Page 841 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 841
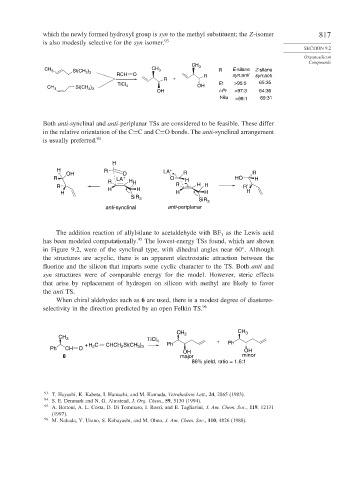
which the newly formed hydroxyl group is syn to the methyl substituent; the Z-isomer 817
is also modestly selective for the syn isomer. 93
SECTION 9.2
Organosilicon
Compounds
CH 3
CH 3 Si(CH ) CH 3 R E-silane Z-silane
3 3
RCH O R syn:anti syn:anti
R +
Et >95:5 65:35
CH 3 Si(CH ) TiCl 4 OH
3 3
OH i-Pr >97:3 64:36
t-Bu >99:1 69:31
Both anti-synclinal and anti-periplanar TSs are considered to be feasible. These differ
in the relative orientation of the C=C and C=O bonds. The anti-synclinal arrangement
is usually preferred. 94
H
H R LA +
OH O R R
R LA + O HO H
R H H H
R H H R H H R
H H H H
SiR 3 SiR 3
anti-synclinal anti-periplanar
The addition reaction of allylsilane to acetaldehyde with BF as the Lewis acid
3
has been modeled computationally. 95 The lowest-energy TSs found, which are shown
in Figure 9.2, were of the synclinal type, with dihedral angles near 60 . Although
the structures are acyclic, there is an apparent electrostatic attraction between the
fluorine and the silicon that imparts some cyclic character to the TS. Both anti and
syn structures were of comparable energy for the model. However, steric effects
that arise by replacement of hydrogen on silicon with methyl are likely to favor
the anti TS.
When chiral aldehydes such as 6 are used, there is a modest degree of diastereo-
selectivity in the direction predicted by an open Felkin TS. 96
CH 3 CH 3
CH 3 TiCl 4
+ H C CHCH 2 Si(CH ) Ph + Ph
Ph CH O 2 3 3
OH OH
6 major minor
86% yield, ratio = 1.6:1
93
T. Hayashi, K. Kabeta, I. Hamachi, and M. Kumada, Tetrahedron Lett., 24, 2865 (1983).
94 S. E. Denmark and N. G. Almstead, J. Org. Chem., 59, 5130 (1994).
95 A. Bottoni, A. L. Costa, D. Di Tommaso, I. Rossi, and E. Tagliavini, J. Am. Chem. Soc., 119, 12131
(1997).
96
M. Nakada, Y. Urano, S. Kobayashi, and M. Ohno, J. Am. Chem. Soc., 110, 4826 (1988).