Page 840 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 840
816 Cl InCl 3
5 mol % InCl OH Si
CHAPTER 9 3
5 mol % (CH ) SiCl O
ArCH O + CHCH Si(CH ) 3 3
Carbon-Carbon CH 2 2 3 3 Ar
Bond-Forming Reactions
of Compounds of Boron,
Silicon, and Tin
Lanthanide salts, such as Sc O SCF , are also effective catalysts. 89
3 3
3
Silylating reagents such as TMSI and TMS triflate have only a modest catalytic
effect, but the still more powerful silylating reagent CH SiB O SCF does induce
3 3 3 3 4
addition to aldehydes. 90
OSi(CH )
(CH ) SiB(O SCF ) 3 3
3
3 4
3 3
RCH O + CH 2 CHCH Si(CH ) RCHCH CH CH 2
2
2
3 3
In another procedure, CH SiN O SCF is generated in situ from triflimide. 91
3 3
3
3
1) 0.5 mol % (CF SO ) NH OH
3 2
3
2) PhCH CH CH O
CH 2 CHCH Si(CH ) 2 2 Ph
2
3 3
90%
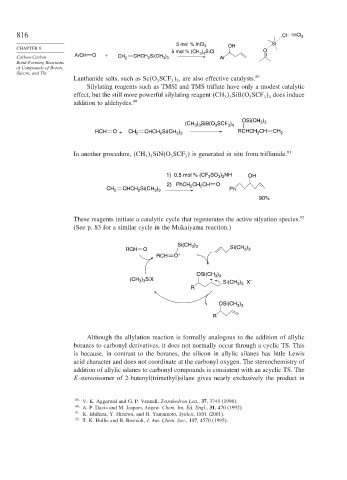
These reagents initiate a catalytic cycle that regenerates the active silyation species. 92
(See p. 83 for a similar cycle in the Mukaiyama reaction.)
Si(CH )
3 3
RCH O Si(CH )
3 3
RCH O +
)
OSi(CH 3 3
) SiX +
(CH 3 3 –
Si(CH )
3 3 X
R
OSi(CH )
3 3
R
Although the allylation reaction is formally analogous to the addition of allylic
boranes to carbonyl derivatives, it does not normally occur through a cyclic TS. This
is because, in contrast to the boranes, the silicon in allylic silanes has little Lewis
acid character and does not coordinate at the carbonyl oxygen. The stereochemistry of
addition of allylic silanes to carbonyl compounds is consistent with an acyclic TS. The
E-stereoisomer of 2-butenyl(trimethyl)silane gives nearly exclusively the product in
89
V. K. Aggarwal and G. P. Vennall, Tetrahedron Lett., 37, 3745 (1996).
90
A. P. Davis and M. Jaspars, Angew. Chem. Int. Ed. Engl., 31, 470 (1992).
91 K. Ishihara, Y. Hiraiwa, and H. Yamamoto, Synlett, 1851 (2001).
92
T. K. Hollis and B. Bosnich, J. Am. Chem. Soc., 117, 4570 (1995).