Page 842 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 842
818
H
CHAPTER 9 Me H Me
Carbon-Carbon H
Bond-Forming Reactions H
of Compounds of Boron,
Silicon, and Tin ω H
O O H
ω
BH 2
Si H 2 B
F
H 3
F SiH 3
ω = 58.6°
ω = 59.8°
1.449
1.690
1.781
1.431 109.0 1.396
1.412
O 1.340
113.8
1.982 1.542 O
132.5
79.0
B 1.509
1.480
B
1.538 122.5
F 1.953 90.4
2.661
Si 1.510
F
2.792
Si
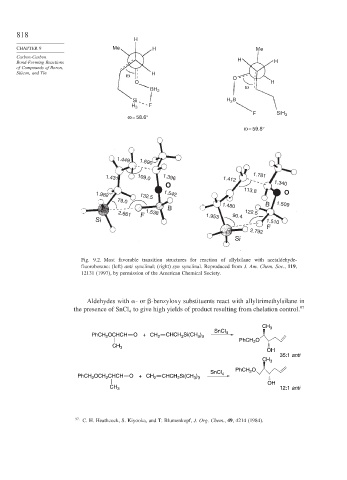
Fig. 9.2. Most favorable transition structures for reaction of allylsilane with acetaldehyde-
fluoroborane: (left) anti synclinal; (right) syn synclinal. Reproduced from J. Am. Chem. Soc., 119,
12131 (1997), by permission of the American Chemical Society.
Aldehydes with -or -benzyloxy substituents react with allyltrimethylsilane in
the presence of SnCl to give high yields of product resulting from chelation control. 97
4
CH 3
SnCl
PhCH OCHCH O + CH 2 CHCH Si(CH ) 4
2
2
3 3
PhCH O
2
CH 3
OH
35:1 anti
CH 3
PhCH O
SnCl 2
PhCH OCH CHCH O + CH 2 CHCH Si(CH ) 4
2
3 3
2
2
OH
CH 3 12:1 anti
97
C. H. Heathcock, S. Kiyooka, and T. Blumenkopf, J. Org. Chem., 49, 4214 (1984).