Page 851 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 851
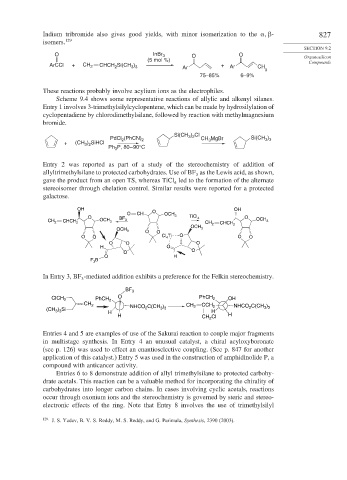
Indium tribromide also gives good yields, with minor isomerization to the
- 827
isomers. 129
SECTION 9.2
O InBr 3 O O Organosilicon
(5 mol %) Compounds
ArCCl + CH 2 CHCH 2 Si(CH ) + Ar CH
Ar
3 3
3
75–85% 6–9%
These reactions probably involve acylium ions as the electrophiles.
Scheme 9.4 shows some representative reactions of allylic and alkenyl silanes.
Entry 1 involves 3-trimethylsilylcyclopentene, which can be made by hydrosilylation of
cyclopentadiene by chlorodimethylsilane, followed by reaction with methylmagnesium
bromide.
) Cl
Si(CH 3 2
3 3
PdCl 2 (PhCN) 2 CH 3 MgBr Si(CH )
+ (CH ) SiHCl
3 2
Ph 3 P, 80 – 90°C
Entry 2 was reported as part of a study of the stereochemistry of addition of
allyltrimethylsilane to protected carbohydrates. Use of BF as the Lewis acid, as shown,
3
gave the product from an open TS, whereas TiCl led to the formation of the alternate
4
stereoisomer through chelation control. Similar results were reported for a protected
galactose.
OH OH
O CH O OCH 3
O BF TiCl 4 O
CH CHCH OCH 3 3 OCH 3
2 2 CH 2 CHCH
OCH 2
OCH 3 O O 3
O O Cl Ti O O O
4
O O O
H O
O O
O H
B
F 3
In Entry 3, BF -mediated addition exhibits a preference for the Felkin stereochemistry.
3
BF 3
O
ClCH 2 PhCH 2 PhCH 2 OH
CH 2 CH 2 CCH 2
(CH 3 ) 3 Si NHCO 2 C(CH 3 ) 3 NHCO 2 C(CH 3 ) 3
H H
H CH 2 Cl H
Entries 4 and 5 are examples of use of the Sakurai reaction to couple major fragments
in multistage synthesis. In Entry 4 an unusual catalyst, a chiral acyloxyboronate
(see p. 126) was used to effect an enantioselective coupling. (See p. 847 for another
application of this catalyst.) Entry 5 was used in the construction of amphidinolide P, a
compound with anticancer activity.
Entries 6 to 8 demonstrate addition of allyl trimethylsilane to protected carbohy-
drate acetals. This reaction can be a valuable method for incorporating the chirality of
carbohydrates into longer carbon chains. In cases involving cyclic acetals, reactions
occur through oxonium ions and the stereochemistry is governed by steric and stereo-
electronic effects of the ring. Note that Entry 8 involves the use of trimethylsilyl
129
J. S. Yadav, B. V. S. Reddy, M. S. Reddy, and G. Parimala, Synthesis, 2390 (2003).