Page 975 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 975
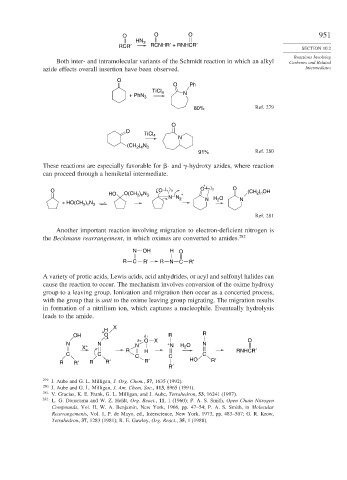
O O O 951
HN 3
RCR′ RCNHR′ + RNHCR′
SECTION 10.2
Reactions Involving
Both inter- and intramolecular variants of the Schmidt reaction in which an alkyl Carbenes and Related
azide effects overall insertion have been observed. Intermediates
O
O Ph
TiCl 4 N
+ PhN 3
80% Ref. 279
O
O TiCl
4
N
) N
(CH 2 4 3
91% Ref. 280
These reactions are especially favorable for - and -hydroxy azides, where reaction
can proceed through a hemiketal intermediate.
+
O O ( ) n O ( ) n O (CH 2 ) n OH
HO O(CH 2 ) n N 3 +
N H 2 O N
N N 2
+ HO(CH 2 ) n N 3
Ref. 281
Another important reaction involving migration to electron-deficient nitrogen is
the Beckmann rearrangement, in which oximes are converted to amides. 282
N OH H O
R C R' R N C R'
A variety of protic acids, Lewis acids, acid anhydrides, or acyl and sulfonyl halides can
cause the reaction to occur. The mechanism involves conversion of the oxime hydroxy
group to a leaving group. Ionization and migration then occur as a concerted process,
with the group that is anti to the oxime leaving group migrating. The migration results
in formation of a nitrilium ion, which captures a nucleophile. Eventually hydrolysis
leads to the amide.
X
+ H
OH O δ+ R R
δ+ O X O
N N N + N H O N
X + 2
R H RNHCR′
C C C C C
R R′ R R′ R′ HO R′
R′
279 J. Aube and G. L. Milligan, J. Org. Chem., 57, 1635 (1992).
280
J. Aube and G. L. Milligan, J. Am. Chem. Soc., 113, 8965 (1991).
281 V. Gracias, K. E. Frank, G. L. Milligan, and J. Aube, Tetrahedron, 53, 16241 (1997).
282
L. G. Donaruma and W. Z. Heldt, Org. React., 11, 1 (1960); P. A. S. Smith, Open Chain Nitrogen
Compounds, Vol. II, W. A. Benjamin, New York, 1966, pp. 47–54; P. A. S. Smith, in Molecular
Rearrangements, Vol. 1, P. de Mayo, ed., Interscience, New York, 1973, pp. 483–507; G. R. Krow,
Tetrahedron, 37, 1283 (1981); R. E. Gawley, Org. React., 35, 1 (1988).