Page 977 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 977
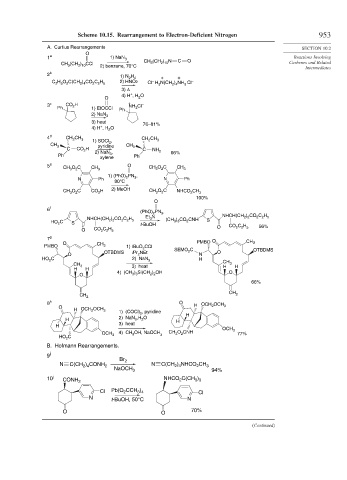
Scheme 10.15. Rearrangement to Electron-Deficient Nitrogen 953
A. Curtius Rearrangements SECTION 10.2
O
1 a 1) NaN 3 Reactions Involving
CH (CH ) N C O
CH (CH ) CCl 2) benzene, 70°C 3 2 10 Carbenes and Related
3
2 10
Intermediates
2 b
1) N H + +
2 4
–
C H O C(CH ) CO C H 2) HNO2 Cl H N(CH ) NH Cl –
2 5
2
2 4
2 2 5
2 4
3
3
3) Δ
+
4) H , H 2 O
O
3 c CO H + NH Cl –
2
Ph 1) EtOCCl Ph 3
2) NaN 3
3) heat 76–81%
+
4) H , H O
2
4 d CH CH 3 1) SOCl , CH CH 3
2
2
2
CH 3 pyridine CH 3
C CO H C
2 , NH 2 66%
Ph 2) NaN 3 Ph
xylene
5 e CH O C CH O O C CH
3 2 3 CH 3 2 3
1) (PhO) PN 3 ,
N Ph 2 N Ph
80°C
CH O C CO H 2) MeOH CH 3 O C CH
3 2 2 2 NHCO 2 3
100%
O
6 f
(PhO) PN , ) CO C H
3
2
) CO C H Et N NHCH(CH 2 2 2 2 5
) CO CNH
NHCH(CH 2 2 2 2 5 3 (CH 3 3 S
C 2
HO 2 S t-BuOH CO C H 56%
O CO C H O 2 2 5
2 2 5
7 g
O PMBO O CH 3
PMBO CH 3 1) i BuO CCl
2
OTBDMS iPr NEt SEMO 2 C O OTBDMS
O 2 N
HO C 2) NaN H
3
2
CH
CH 3 3) heat H 3 H
H H
3 3
O 4) (CH ) Si(CH ) OH O
2 2
66%
CH
CH 2 2
8 h O H OCH OCH
O 2 3
H OCH OCH
3
2
1) (COCl) , pyridine
2
2) NaN ,H O H
H 3 2 H
3) heat
H
OCH
4) CH OH, NaOCH CH 3 O CNH 3
OCH 3 3 3 2 77%
C
HO 2
B. Hofmann Rearrangements.
9 i
Br 2
N C(CH 2 4 2 N C(CH ) NHCO CH 3
) CONH
2
2 4
NaOCH 3 94%
10 j CONH 2 NHCO C(CH )
2
3 3
Cl Pb(O CCH ) Cl
3 4
2
N t-BuOH, 50°C N
O O 70%
(Continued)