Page 974 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 974
950 Phenyliodonium diacetate, 274
275 and phenyliodonium bis-trifluoroacetate, 276 are also
useful oxidants for converting amides to carbamates.
CHAPTER 10
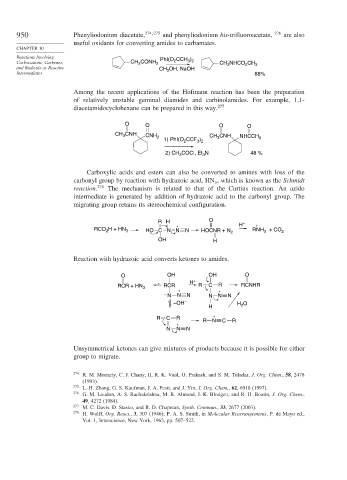
Reactions Involving PhI(O CCH )
Carbocations, Carbenes, CH CONH 2 2 3 2 CH NHCO CH 3
2
2
2
and Radicals as Reactive OH, NaOH
CH 3
Intermediates 88%
Among the recent applications of the Hofmann reaction has been the preparation
of relatively unstable geminal diamides and carbinolamides. For example, 1,1-
diacetamidocyclohexane can be prepared in this way. 277
O O O O
CH CNH CNH 2 CH CNH
3
1) PhI(O CCF ) 3 NHCCH 3
2
3 2
2) CH COCl, Et N 48 %
3
3
Carboxylic acids and esters can also be converted to amines with loss of the
carbonyl group by reaction with hydrazoic acid, HN , which is known as the Schmidt
3
reaction. 278 The mechanism is related to that of the Curtius reaction. An azido
intermediate is generated by addition of hydrazoic acid to the carbonyl group. The
migrating group retains its stereochemical configuration.
R H O +
+ H +
RCO H + HN 3 HO CN N N HOCNR + N 2 RNH + CO 2
2
3
OH H
Reaction with hydrazoic acid converts ketones to amides.
O OH OH O
H +
RCR + HN 3 RCR R C R RCNHR
+ +
– N N N N N N
–OH – H O
H 2
R C R R N + C R
+
N N N
Unsymmetrical ketones can give mixtures of products because it is possible for either
group to migrate.
274 R. M. Moriarty, C. J. Chany, II, R. K. Vaid, O. Prakash, and S. M. Tuladar, J. Org. Chem., 58, 2478
(1993).
275
L.-H. Zhang, G. S. Kaufman, J. A. Pesti, and J. Yin, J. Org. Chem., 62, 6918 (1997).
276
G. M. Loudon, A. S. Radhakrishna, M. R. Almond, J. K. Blodgett, and R. H. Boutin, J. Org. Chem.,
49, 4272 (1984).
277 M. C. Davis, D. Stasko, and R. D. Chapman, Synth. Commun., 33, 2677 (2003).
278
H. Wolff, Org. React., 3, 307 (1946); P. A. S. Smith, in Molecular Rearrangements, P. de Mayo ed.,
Vol. 1, Interscience, New York, 1963, pp. 507–522.