Page 985 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 985
.
the addition step k is not fast enough, the radical R will abstract H from the stannane 961
1
and the overall reaction will simply be dehalogenation. If step k is not fast relative
2
to a successive addition step, formation of oligomers containing several alkene units SECTION 10.3
. Reactions Involving Free
will occur. For good yields R must be more reactive to the substituted alkene than is Radical Intermediates
.
.
.
RCH C HZ and RCH C HZ must be more reactive toward Bu SnH than is R . These
2
3
2
requirements are met when Z is an electron-attracting group. Yields are also improved
if the concentration of Bu SnH is kept low to minimize the reductive dehalogenation,
3
which can be done by adding the stannane slowly as the reaction proceeds. Another
method is to use only a small amount of the trialkyltin hydride along with a reducing
agent, such as NaBH or NaBH CN, that can regenerate the reactive stannane. 303
4
3
Radicals formed by fragmentation of thionocarbonates and related thiono esters can
also be trapped by reactive alkenes. The mechanism of radical generation from
thiono esters was discussed in connection with the Barton deoxygenation method in
Section 5.5.
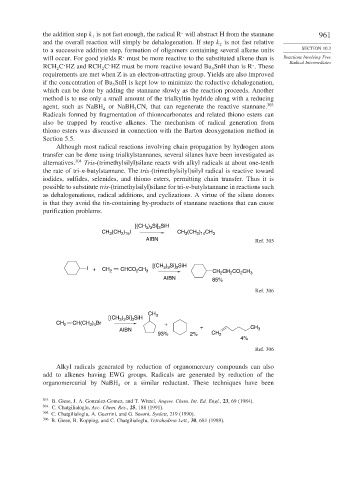
Although most radical reactions involving chain propagation by hydrogen atom
transfer can be done using trialkylstannanes, several silanes have been investigated as
alternatives. 304 Tris-(trimethylsilyl)silane reacts with alkyl radicals at about one-tenth
the rate of tri-n-butylstannane. The tris-(trimethylsilyl)silyl radical is reactive toward
iodides, sulfides, selenides, and thiono esters, permitting chain transfer. Thus it is
possible to substitute tris-(trimethylsilyl)silane for tri-n-butylstannane in reactions such
as dehalogenations, radical additions, and cyclizations. A virtue of the silane donors
is that they avoid the tin-containing by-products of stannane reactions that can cause
purification problems.
[(CH ) Si] SiH
3
3 3
CH (CH ) I CH (CH ) CH 3
3
2 15
3
2 14
AIBN Ref. 305
[(CH ) Si] SiH
I + CH 2 CHCO 2 CH 3 3 3 3 CH CH CO CH 3
2
2
2
AIBN 85%
Ref. 306
CH
[(CH ) Si] SiH 3
3
3 3
CH 2 CH(CH 2 ) 4 Br +
AIBN + CH 3
93% 2% CH 2
4%
Ref. 306
Alkyl radicals generated by reduction of organomercury compounds can also
add to alkenes having EWG groups. Radicals are generated by reduction of the
organomercurial by NaBH or a similar reductant. These techniques have been
4
303
B. Giese, J. A. Gonzalez-Gomez, and T. Witzel, Angew. Chem. Int. Ed. Engl., 23, 69 (1984).
304 C. Chatgilialoglu, Acc. Chem. Res., 25, 188 (1991).
305 C. Chatgilialoglu, A. Guerrini, and G. Sesoni, Synlett, 219 (1990).
306
B. Giese, B. Kopping, and C. Chatgilialoglu, Tetrahedron Lett., 30, 681 (1989).