Page 990 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 990
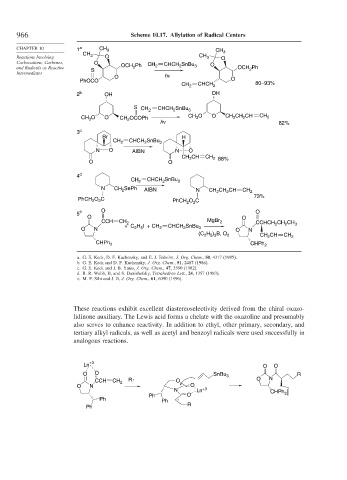
966 Scheme 10.17. Allylation of Radical Centers
CHAPTER 10 1 a CH 3 CH 3
CH 3 CH
Reactions Involving O 3 O
Carbocations, Carbenes, O CH SnBu O
2
and Radicals as Reactive S OCH Ph 2 CHCH 2 3 OCH Ph
2
Intermediates hν
O
PhOCO O
CH 2 CHCH 2 80–93%
2 b OH OH
CHCH SnBu
S CH 2 2 3
CH O O OCOPh CH 3 O O CH 2 CH 2 CH CH 2
3
hν 82%
CH 2
3 c
Br H
CH 2 CHCH 2 SnBu 3
N O AIBN N O
CH CH CH 2 88%
2
O O
4 d
CH 2 CHCH SnBu 3
2
N CH 2 SePh AIBN N CH CH CH CH 2
2
2
73%
O C
PhCH 2 2 PhCH O C
2
2
5 e O O
O O
CCH CH 2 MgBr 2 CCHCH CH CH
+ C H I + CH CHCH SnBu 2 2 3
O N 2 5 2 2 3 O N
(C H ) B, O 2 CH CH CH 2
2 5 3
2
CHPh 2 CHPh 2
a. G. E. Keck, D. F. Kachensky, and E. J. Enholm, J. Org. Chem., 50, 4317 (1985).
b. G. E. Keck and D. F. Kachensky, J. Org. Chem., 51, 2487 (1986).
c. G. E. Keck and J. B. Yates, J. Org. Chem., 47, 3590 (1982).
d. R. R. Webb, II, and S. Danishefsky, Tetrahedron Lett., 24, 1357 (1983).
e. M. P. Sibi and J. Ji, J. Org. Chem., 61, 6090 (1996).
These reactions exhibit excellent diastereoselectivity derived from the chiral oxazo-
lidinone auxiliary. The Lewis acid forms a chelate with the oxazoline and presumably
also serves to enhance reactivity. In addition to ethyl, other primary, secondary, and
tertiary alkyl radicals, as well as acetyl and benzoyl radicals were used successfully in
analogous reactions.
Ln +3 O O
O O SnBu 3 R
CCH CH 2 R . O O N
O N O
N Ln +3
Ph O CHPh 2
Ph Ph .
Ph R