Page 992 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 992
968
CHAPTER 10
Reactions Involving
Carbocations, Carbenes,
and Radicals as Reactive
Intermediates
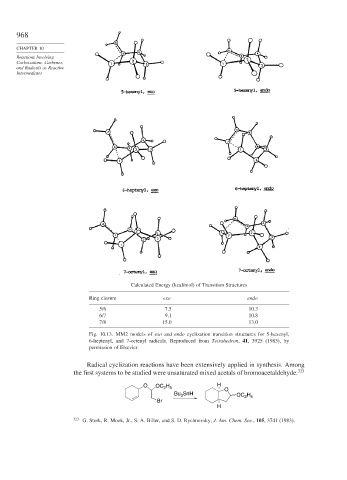
Calculated Energy (kcal/mol) of Transition Structures
Ring closure exo endo
5/6 7.5 10.3
6/7 9.1 10.8
7/8 15.0 13.0
Fig. 10.13. MM2 models of exo and endo cyclization transition structures for 5-hexenyl,
6-heptenyl, and 7-octenyl radicals. Reproduced from Tetrahedron, 41, 3925 (1985), by
permission of Elsevier.
Radical cyclization reactions have been extensively applied in synthesis. Among
the first systems to be studied were unsaturated mixed acetals of bromoacetaldehyde. 323
O OC H H O
2 5
SnH
Bu 3 H
OC 2 5
Br
H
323
G. Stork, R. Mook, Jr., S. A. Biller, and S. D. Rychnovsky, J. Am. Chem. Soc., 105, 3741 (1983).