Page 996 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 996
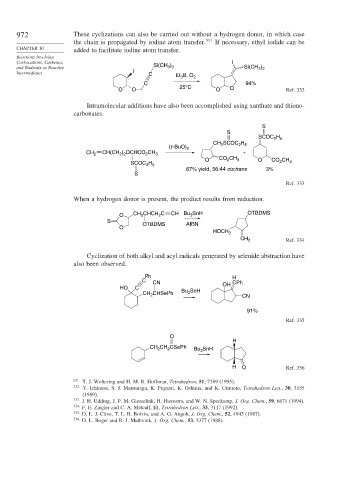
972 These cyclizations can also be carried out without a hydrogen donor, in which case
the chain is propagated by iodine atom transfer. 331 If necessary, ethyl iodide can be
CHAPTER 10 added to facilitate iodine atom transfer.
Reactions Involving
Carbocations, Carbenes, ) I
)
and Radicals as Reactive Si(CH 3 3 Si(CH 3 3
Intermediates I C Et B, O 2
3
C 94%
O O 25°C O O Ref. 332
Intramolecular additions have also been accomplished using xanthate and thiono-
carbonates.
S
S
SCOC H
2 5
CH SCOC H
(t -BuO) 2 2 2 5
CH(CH ) OCHCO CH +
CH 2 2 2 2 3
O CO CH 3 O CO CH
2
SCOC H 2 3
2 5
67% yield, 56:44 cis:trans 3%
S
Ref. 333
When a hydrogen donor is present, the product results from reduction.
3
O CH 2 CHCH C CH Bu SnH OTBDMS
2
S OTBDMS AIBN
O
HOCH 2
Ref. 334
CH 2
Cyclization of both alkyl and acyl radicals generated by selenide abstraction have
also been observed.
Ph H
C
CN OH CPh
HO C SnH
CH 2 CHSePh Bu 3
CN
91%
Ref. 335
O
H
CH CSePh
CH 2 2 Bu 3 SnH
H O Ref. 336
331
T. J. Woltering and H. M. R. Hoffman, Tetrahedron, 51, 7389 (1995).
332 Y. Ichinose, S. J. Matsunaga, K. Fugami, K. Oshima, and K. Utimoto, Tetrahedron Lett., 30, 3155
(1989).
333
J. H. Udding, J. P. M. Giesselink, H. Hiemstra, and W. N. Speckamp, J. Org. Chem., 59, 6671 (1994).
334 F. E. Ziegler and C. A. Metcalf, III, Tetrahedron Lett., 33, 3117 (1992).
335 D. L. J. Clive, T. L. B. Boivin, and A. G. Angoh, J. Org. Chem., 52, 4943 (1987).
336
D. L. Boger and R. J. Mathvink, J. Org. Chem., 53, 3377 (1988).