Page 999 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 999
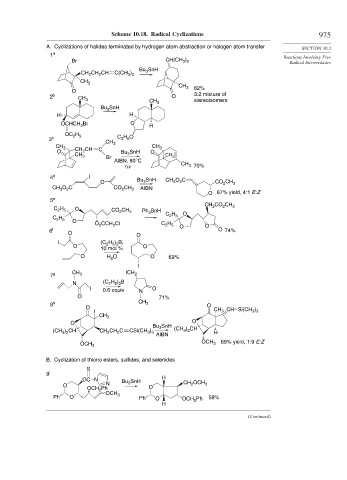
Scheme 10.18. Radical Cyclizations 975
A. Cyclizations of halides terminated by hydrogen atom abstraction or halogen atom transfer
SECTION 10.3
1 a Reactions Involving Free
Br CH(CH ) Radical Intermediates
3 2
Bu SnH
CH CH CH C(CH ) 3
3 2
2
2
CH 3
CH 3
O 62%
2 b CH 3 CH O 3:2 mixture of
stereoisomers
Bu 3 SnH 3
H H
OCHCH Br O H
2
OC H
2 5
2 5
3 c C H O
CH 3
CH 3 CH CH CH 3
O 2 C SnH O
CH 3 Br Bu 3 CH 3
AIBN, 80°C CH
hv 3 70%
4 d I SnH
3
2
O Bu 3 CH O C CO CH 3
2
CH O C CO CH 3 AIBN
2
3
2
O 87% yield, 4:1 E:Z
5 e
CH CO CH
C H O CO CH 3 Ph 3 SnH 2 2 3
2 5
2
2 5
C H C H O
2 5
O
O CCH Cl C 2 5 O O
H
2
2
6 f O O O 74%
I (C H ) B,
2 5 3
O 10 mol % O
O H O O 69%
2
I
7 g CH 3 ICH 2
H ) B
N (C 2 5 3
I 0.6 equiv N O
O 71%
8 h CH 3 O
O )
I CH CH Si(CH 3 3
3
CH 3
O O
Bu SnH
3
(CH ) CH CH CH C CSi(CH ) AIBN (CH ) CH H
3 2
2
3 2
2
3 3
OCH 69% yield, 1:9 E:Z
OCH 3 3
B. Cyclization of thiono esters, sulfides, and selenides
S
9 i
OC N Bu SnH H CH OCH
O N 3 O 2 3
OCH Ph
2
OCH
Ph O 3 Ph O OCH 2 Ph 58%
H
(Continued)