Page 1002 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1002
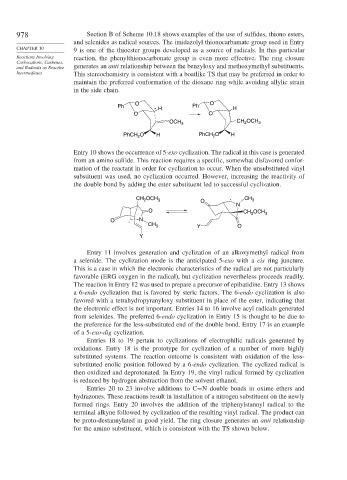
978 Section B of Scheme 10.18 shows examples of the use of sulfides, thiono esters,
and selenides as radical sources. The imidazolyl thionocarbamate group used in Entry
CHAPTER 10 9 is one of the thioester groups developed as a source of radicals. In this particular
Reactions Involving reaction, the phenylthionocarbonate group is even more effective. The ring closure
Carbocations, Carbenes,
and Radicals as Reactive generates an anti relationship between the benzyloxy and methoxymethyl substituents.
Intermediates This stereochemistry is consistent with a boatlike TS that may be preferred in order to
maintain the preferred conformation of the dioxane ring while avoiding allylic strain
in the side chain.
O O
Ph . H Ph H
O O
OCH 3 CH OCH 3
2
PhCH 2 O H PhCH 2 O H
Entry 10 shows the occurrence of 5-exo cyclization. The radical in this case is generated
from an amino sulfide. This reaction requires a specific, somewhat disfavored confor-
mation of the reactant in order for cyclization to occur. When the unsubstituted vinyl
substituent was used, no cyclization occurred. However, increasing the reactivity of
the double bond by adding the ester substituent led to successful cyclization.
CH OCH 3 O . CH 3
2
N
O CH 2 OCH 3
O . N
Y O
CH 3
Y
Entry 11 involves generation and cyclization of an alkoxymethyl radical from
a selenide. The cyclization mode is the anticipated 5-exo with a cis ring juncture.
This is a case in which the electronic characteristics of the radical are not particularly
favorable (ERG oxygen in the radical), but cyclization nevertheless proceeds readily.
The reaction in Entry 12 was used to prepare a precursor of epibatidine. Entry 13 shows
a6-endo cyclization that is favored by steric factors. The 6-endo cyclization is also
favored with a tetrahydropyranyloxy substituent in place of the ester, indicating that
the electronic effect is not important. Entries 14 to 16 involve acyl radicals generated
from selenides. The preferred 6-endo cyclization in Entry 15 is thought to be due to
the preference for the less-substituted end of the double bond. Entry 17 is an example
ofa5-exo-dig cyclization.
Entries 18 to 19 pertain to cyclizations of electrophilic radicals generated by
oxidations. Entry 18 is the prototype for cyclization of a number of more highly
substituted systems. The reaction outcome is consistent with oxidation of the less-
substituted enolic position followed by a 6-endo cyclization. The cyclized radical is
then oxidized and deprotonated. In Entry 19, the vinyl radical formed by cyclization
is reduced by hydrogen abstraction from the solvent ethanol.
Entries 20 to 23 involve additions to C=N double bonds in oxime ethers and
hydrazones. These reactions result in installation of a nitrogen substituent on the newly
formed rings. Entry 20 involves the addition of the triphenylstannyl radical to the
terminal alkyne followed by cyclization of the resulting vinyl radical. The product can
be proto-destannylated in good yield. The ring closure generates an anti relationship
for the amino substituent, which is consistent with the TS shown below.