Page 995 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 995
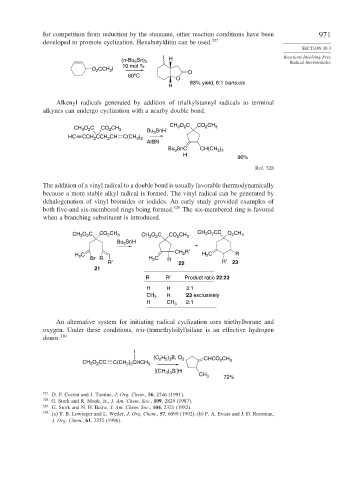
for competition from reduction by the stannane, other reaction conditions have been 971
developed to promote cyclization. Hexabutylditin can be used. 327
SECTION 10.3
I Reactions Involving Free
(n-Bu Sn) 2 H Radical Intermediates
3
10 mol %
O CCH I O
2
2
80°C
O
83% yield, 6:1 trans:cis
H
Alkenyl radicals generated by addition of trialkylstannyl radicals to terminal
alkynes can undergo cyclization with a nearby double bond.
CH O C CO CH
CH O C CO CH 3 Bu SnH 3 2 2 3
2
3
2
HC CCH 2 CCH 2 CH C(CH 3 ) 2 3
AIBN
3 2
Bu 3 SnC CH(CH )
H
90%
Ref. 328
The addition of a vinyl radical to a double bond is usually favorable thermodynamically
because a more stable alkyl radical is formed. The vinyl radical can be generated by
dehalogenation of vinyl bromides or iodides. An early study provided examples of
both five-and six-membered rings being formed. 329 The six-membered ring is favored
when a branching substituent is introduced.
2
3
2
2
CH 3 O C CO CH 3 CH O C CO CH 3 CH O CC O 2 CH 3
2
3
2
Bu SnH
3
+
CH R′
H C Br R H C 2 H C R
2
2
R′ 2 R 22 R′ 23
21
R R′ Product ratio 22:23
H H 3:1
H 23 exclusively
CH 3
H CH 3 2:1
An alternative system for initiating radical cyclization uses triethylborane and
oxygen. Under these conditions, tris-(trimethylsilyl)silane is an effective hydrogen
donor. 330
I
H ) B, O
(C 2 5 3 2 CHCO 2 CH 3
CH O CC C(CH ) CHCH 3
2
3
2 3
[(CH ) Si]H CH 3 72%
3 3
327 D. P. Curran and J. Tamine, J. Org. Chem., 56, 2746 (1991).
328
G. Stork and R. Mook, Jr., J. Am. Chem. Soc., 109, 2829 (1987).
329 G. Stork and N. H. Baine, J. Am. Chem. Soc., 104, 2321 (1982).
330
(a) T. B. Lowinger and L. Weiler, J. Org. Chem., 57, 6099 (1992); (b) P. A. Evans and J. D. Roseman,
J. Org. Chem., 61, 2252 (1996).