Page 133 - Advanced Thermodynamics for Engineers, Second Edition
P. 133
120 CHAPTER 6 FINITE TIME (OR ENDOREVERSIBLE) THERMODYNAMICS
T H
.
Q H
.
T 1 W
E
T 2
.
Q C
T C
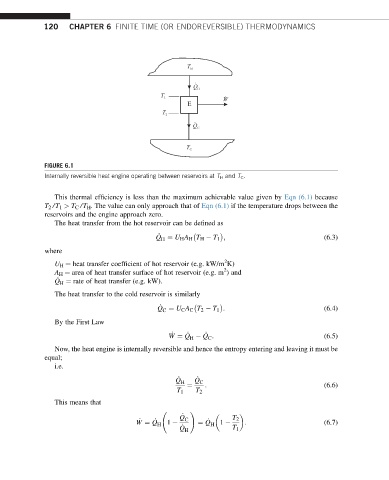
FIGURE 6.1
Internally reversible heat engine operating between reservoirs at T H and T C .
This thermal efficiency is less than the maximum achievable value given by Eqn (6.1) because
T 2 /T 1 > T C /T H . The value can only approach that of Eqn (6.1) if the temperature drops between the
reservoirs and the engine approach zero.
The heat transfer from the hot reservoir can be defined as
_
Q ¼ U H A H T H T 1 ; (6.3)
H
where
2
U H ¼ heat transfer coefficient of hot reservoir (e.g. kW/m K)
2
A H ¼ area of heat transfer surface of hot reservoir (e.g. m ) and
_
Q ¼ rate of heat transfer (e.g. kW).
H
The heat transfer to the cold reservoir is similarly
_
Q ¼ U C A C T 2 T 1 : (6.4)
C
By the First Law
_
_
_
W ¼ Q Q : (6.5)
C
H
Now, the heat engine is internally reversible and hence the entropy entering and leaving it must be
equal;
i.e.
Q _ H Q _ C
¼ : (6.6)
T 1 T 2
This means that
!
Q _ T 2
_
W ¼ Q _ H 1 C ¼ Q _ H 1 : (6.7)
Q _ H T 1