Page 311 - Advanced Thermodynamics for Engineers, Second Edition
P. 311
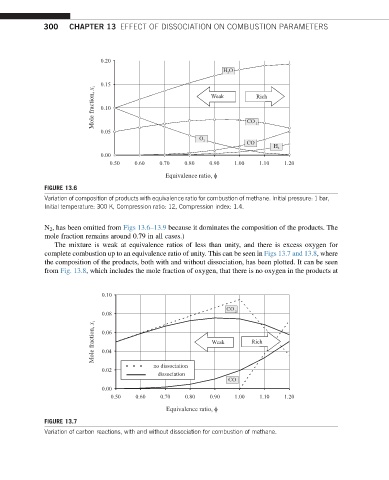
300 CHAPTER 13 EFFECT OF DISSOCIATION ON COMBUSTION PARAMETERS
0.20
HO
0.15
Mole fraction, x i 0.10 Weak Rich
0.05 CO
O
CO
H
0.00
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20
Equivalence ratio, φ
FIGURE 13.6
Variation of composition of products with equivalence ratio for combustion of methane. Initial pressure: 1 bar,
Initial temperature: 300 K, Compression ratio: 12, Compression index: 1.4.
N 2 , has been omitted from Figs 13.6–13.9 because it dominates the composition of the products. The
mole fraction remains around 0.79 in all cases.)
The mixture is weak at equivalence ratios of less than unity, and there is excess oxygen for
complete combustion up to an equivalence ratio of unity. This can be seen in Figs 13.7 and 13.8, where
the composition of the products, both with and without dissociation, has been plotted. It can be seen
from Fig. 13.8, which includes the mole fraction of oxygen, that there is no oxygen in the products at
0.10
CO
0.08
Mole fraction, x i 0.06 Weak Rich
0.04
no dissociation
0.02
dissociation
CO
0.00
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20
Equivalence ratio, φ
FIGURE 13.7
Variation of carbon reactions, with and without dissociation for combustion of methane.