Page 309 - Advanced Thermodynamics for Engineers, Second Edition
P. 309
298 CHAPTER 13 EFFECT OF DISSOCIATION ON COMBUSTION PARAMETERS
140
120
100 Weak Rich
Pressure / (bar) 80
60
40
no dissociation
dissociation
20
0
0.5 0.6 0.7 0.8 0.9 1 1.1 1.2 1.3
Equivalence ratio, φ
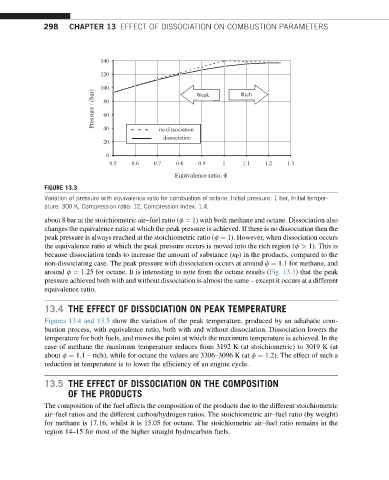
FIGURE 13.3
Variation of pressure with equivalence ratio for combustion of octane. Initial pressure: 1 bar, Initial temper-
ature: 300 K, Compression ratio: 12, Compression index: 1.4.
about 8 bar at the stoichiometric air–fuel ratio (f ¼ 1) with both methane and octane. Dissociation also
changes the equivalence ratio at which the peak pressure is achieved. If there is no dissociation then the
peak pressure is always reached at the stoichiometric ratio (f ¼ 1). However, when dissociation occurs
the equivalence ratio at which the peak pressure occurs is moved into the rich region (f > 1). This is
because dissociation tends to increase the amount of substance (n P ) in the products, compared to the
non-dissociating case. The peak pressure with dissociation occurs at around f ¼ 1.1 for methane, and
around f ¼ 1.25 for octane. It is interesting to note from the octane results (Fig. 13.3) that the peak
pressure achieved both with and without dissociation is almost the same – except it occurs at a different
equivalence ratio.
13.4 THE EFFECT OF DISSOCIATION ON PEAK TEMPERATURE
Figures 13.4 and 13.5 show the variation of the peak temperature, produced by an adiabatic com-
bustion process, with equivalence ratio, both with and without dissociation. Dissociation lowers the
temperature for both fuels, and moves the point at which the maximum temperature is achieved. In the
case of methane the maximum temperature reduces from 3192 K (at stoichiometric) to 3019 K (at
about f ¼ 1.1 – rich), while for octane the values are 3306–3096 K (at f ¼ 1.2). The effect of such a
reduction in temperature is to lower the efficiency of an engine cycle.
13.5 THE EFFECT OF DISSOCIATION ON THE COMPOSITION
OF THE PRODUCTS
The composition of the fuel affects the composition of the products due to the different stoichiometric
air–fuel ratios and the different carbon/hydrogen ratios. The stoichiometric air–fuel ratio (by weight)
for methane is 17.16, whilst it is 15.05 for octane. The stoichiometric air–fuel ratio remains in the
region 14–15 for most of the higher straight hydrocarbon fuels.