Page 343 - Advanced thermodynamics for engineers
P. 343
332 CHAPTER 15 COMBUSTION AND FLAMES
Stoichiometric Stoichiometric Stoichiometric
C H CH H , CO
250
H
Stoichiometric
Laminar flame speed / (cm/s) 150 CH CH
C H
200
100
50
0 CH CO
0 10 20 30 40 50 60 70
% gas in air
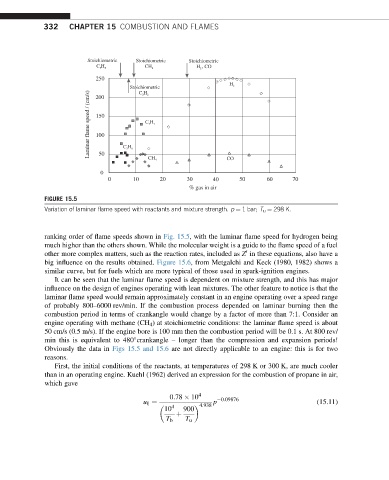
FIGURE 15.5
Variation of laminar flame speed with reactants and mixture strength. p ¼ 1 bar; T u ¼ 298 K.
ranking order of flame speeds shown in Fig. 15.5, with the laminar flame speed for hydrogen being
much higher than the others shown. While the molecular weight is a guide to the flame speed of a fuel
other more complex matters, such as the reaction rates, included as Z in these equations, also have a
0
big influence on the results obtained. Figure 15.6, from Metgalchi and Keck (1980, 1982) shows a
similar curve, but for fuels which are more typical of those used in spark-ignition engines.
It can be seen that the laminar flame speed is dependent on mixture strength, and this has major
influence on the design of engines operating with lean mixtures. The other feature to notice is that the
laminar flame speed would remain approximately constant in an engine operating over a speed range
of probably 800–6000 rev/min. If the combustion process depended on laminar burning then the
combustion period in terms of crankangle would change by a factor of more than 7:1. Consider an
engine operating with methane (CH 4 ) at stoichiometric conditions: the laminar flame speed is about
50 cm/s (0.5 m/s). If the engine bore is 100 mm then the combustion period will be 0.1 s. At 800 rev/
min this is equivalent to 480 crankangle – longer than the compression and expansion periods!
Obviously the data in Figs 15.5 and 15.6 are not directly applicable to an engine: this is for two
reasons.
First, the initial conditions of the reactants, at temperatures of 298 K or 300 K, are much cooler
than in an operating engine. Kuehl (1962) derived an expression for the combustion of propane in air,
which gave
0:78 10 4
u [ ¼ p 0:09876 (15.11)
4 4:938
10 900
þ
T b T u