Page 344 - Advanced thermodynamics for engineers
P. 344
15.4 FLAMES 333
50
45
Methanol
Laminar flame speed / (cm/s) 35 Iso-octane Propane
40
30
25
Petrol
Methane
20
Lean Rich
15
0.8 0.9 1.0 1.1 1.2 1.3 1.4 1.5
Fuel-air equivalence ratio
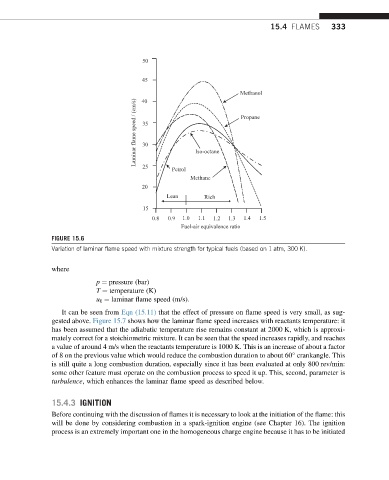
FIGURE 15.6
Variation of laminar flame speed with mixture strength for typical fuels (based on 1 atm, 300 K).
where
p ¼ pressure (bar)
T ¼ temperature (K)
u [ ¼ laminar flame speed (m/s).
It can be seen from Eqn (15.11) that the effect of pressure on flame speed is very small, as sug-
gested above. Figure 15.7 shows how the laminar flame speed increases with reactants temperature: it
has been assumed that the adiabatic temperature rise remains constant at 2000 K, which is approxi-
mately correct for a stoichiometric mixture. It can be seen that the speed increases rapidly, and reaches
a value of around 4 m/s when the reactants temperature is 1000 K. This is an increase of about a factor
of 8 on the previous value which would reduce the combustion duration to about 60 crankangle. This
is still quite a long combustion duration, especially since it has been evaluated at only 800 rev/min:
some other feature must operate on the combustion process to speed it up. This, second, parameter is
turbulence, which enhances the laminar flame speed as described below.
15.4.3 IGNITION
Before continuing with the discussion of flames it is necessary to look at the initiation of the flame: this
will be done by considering combustion in a spark-ignition engine (see Chapter 16). The ignition
process is an extremely important one in the homogeneous charge engine because it has to be initiated