Page 529 - Advanced thermodynamics for engineers
P. 529
522 CHAPTER 21 FUEL CELLS
21.5.5 OVERALL RESPONSE
The overall response is derived using a combination of these loss terms, giving:
i þ i n i þ i n
V ¼ E i þ i n r A ln þ B ln 1 (21.66)
i 0 i l
The high capital cost and bulk of fuel cells mean that they are frequently operated at the maximum
power density, giving a cell voltage of about 0.6 V for both SPFCs and SOFCs. The reasons for the
voltage losses are quite different for the two types of cell. The SOFC has a high Ohmic loss, but a low
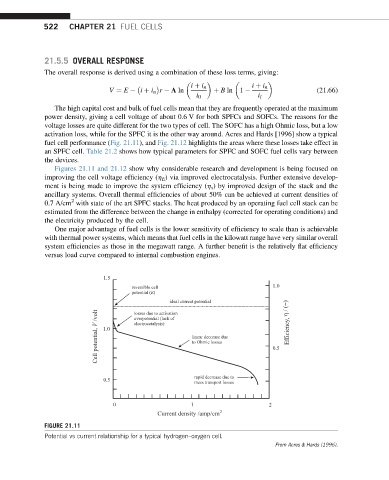
activation loss, while for the SPFC it is the other way around. Acres and Hards [1996] show a typical
fuel cell performance (Fig. 21.11), and Fig. 21.12 highlights the areas where these losses take effect in
an SPFC cell. Table 21.2 shows how typical parameters for SPFC and SOFC fuel cells vary between
the devices.
Figures 21.11 and 21.12 show why considerable research and development is being focused on
improving the cell voltage efficiency (h E ) via improved electrocatalysis. Further extensive develop-
ment is being made to improve the system efficiency (h s ) by improved design of the stack and the
ancillary systems. Overall thermal efficiencies of about 50% can be achieved at current densities of
2
0.7 A/cm with state of the art SPFC stacks. The heat produced by an operating fuel cell stack can be
estimated from the difference between the change in enthalpy (corrected for operating conditions) and
the electricity produced by the cell.
One major advantage of fuel cells is the lower sensitivity of efficiency to scale than is achievable
with thermal power systems, which means that fuel cells in the kilowatt range have very similar overall
system efficiencies as those in the megawatt range. A further benefit is the relatively flat efficiency
versus load curve compared to internal combustion engines.
1.5
reversible cell 1.0
potential (E)
ideal current potential
Cell potential, V /volt 1.0 electrocatalysis) linear decrease due 0.5 Efficiency,
losses due to activation
overpotential (lack of
to Ohmic losses
rapid decrease due to
0.5 mass transport losses
0 1 2
Current density /amp/cm 2
FIGURE 21.11
Potential vs current relationship for a typical hydrogen–oxygen cell.
From Acres & Hards (1996).