Page 526 - Advanced thermodynamics for engineers
P. 526
21.5 LOSSES IN FUEL CELLS 519
1.0
Measured
voltage
Modelled
voltage
Voltage 0.5 A
log i 0
0.0
1x10 -6 1x10 -5 1x10 -4 1x10 -3
logarithm of current (log i)
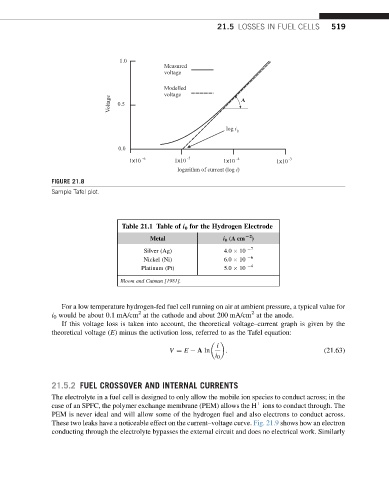
FIGURE 21.8
Sample Tafel plot.
Table 21.1 Table of i 0 for the Hydrogen Electrode
Metal i 0 (A cm L2 )
Silver (Ag) 4.0 10 7
Nickel (Ni) 6.0 10 6
Platinum (Pt) 5.0 10 4
Bloom and Cutman [1981].
For a low temperature hydrogen-fed fuel cell running on air at ambient pressure, a typical value for
2
2
i 0 would be about 0.1 mA/cm at the cathode and about 200 mA/cm at the anode.
If this voltage loss is taken into account, the theoretical voltage–current graph is given by the
theoretical voltage (E) minus the activation loss, referred to as the Tafel equation:
i
V ¼ E A ln : (21.63)
i 0
21.5.2 FUEL CROSSOVER AND INTERNAL CURRENTS
The electrolyte in a fuel cell is designed to only allow the mobile ion species to conduct across; in the
case of an SPFC, the polymer exchange membrane (PEM) allows the H ions to conduct through. The
þ
PEM is never ideal and will allow some of the hydrogen fuel and also electrons to conduct across.
These two leaks have a noticeable effect on the current–voltage curve. Fig. 21.9 shows how an electron
conducting through the electrolyte bypasses the external circuit and does no electrical work. Similarly