Page 530 - Advanced thermodynamics for engineers
P. 530
21.6 SOURCES OF HYDROGEN FOR FUEL CELLS 523
open circuit potential
Cell potential, V/volt 1.0 cell potential membrane Ohmic
cathode activation overpotential
anode activation
0.5
0.5 1.0
Current density / amp/cm 2
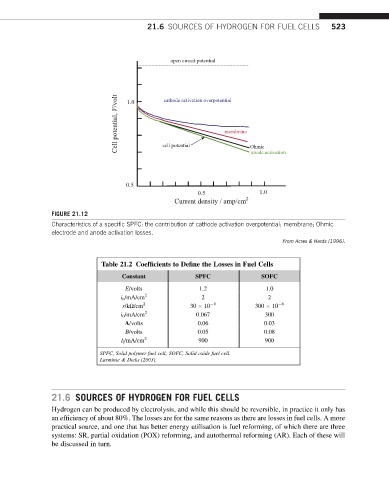
FIGURE 21.12
Characteristics of a specific SPFC: the contribution of cathode activation overpotential; membrane; Ohmic
electrode and anode activation losses.
From Acres & Hards (1996).
Table 21.2 Coefficients to Define the Losses in Fuel Cells
Constant SPFC SOFC
E/volts 1.2 1.0
i n /mA/cm 2 2 2
r/kU/cm 2 30 10 6 300 10 6
i 0 /mA/cm 2 0.067 300
A/volts 0.06 0.03
B/volts 0.05 0.08
I l /mA/cm 2 900 900
SPFC, Solid polymer fuel cell; SOFC, Solid oxide fuel cell.
Larminie & Dicks (2003).
21.6 SOURCES OF HYDROGEN FOR FUEL CELLS
Hydrogen can be produced by electrolysis, and while this should be reversible, in practice it only has
an efficiency of about 80%. The losses are for the same reasons as there are losses in fuel cells. A more
practical source, and one that has better energy utilisation is fuel reforming, of which there are three
systems: SR, partial oxidation (POX) reforming, and autothermal reforming (AR). Each of these will
be discussed in turn.