Page 127 - Advances in Biomechanics and Tissue Regeneration
P. 127
7.2 EQUATIONS THAT GOVERN THE ELECTRICAL ACTIVITY OF THE HEART 123
7.2.3.5 The Ten Tusscher Action Potential Model
Modern AP models incorporate many of the features formerly described and their formulation is reduced to
Eq. (7.29) in addition to differential equations (7.27) governing the dynamics of the gates and the ionic concentrations
in the cytoplasm. The formulation of these models is based on experimental data collected from different animal spe-
cies. They are, in general, relatively costly from a computational point of view due to the nonlinearity of their equa-
tions. Among the most widely used models of AP for human ventricle is the one proposed by ten Tusscher et al. [8, 41]
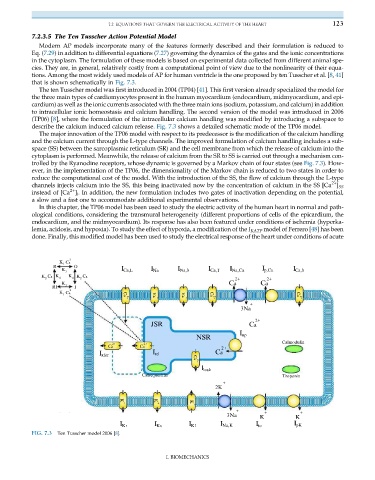
that is shown schematically in Fig. 7.3.
The ten Tusscher model was first introduced in 2004 (TP04) [41]. This first version already specialized the model for
the three main types of cardiomyocytes present in the human myocardium (endocardium, midmyocardium, and epi-
cardium) as well as the ionic currents associated with the three main ions (sodium, potassium, and calcium) in addition
to intracellular ionic homeostasis and calcium handling. The second version of the model was introduced in 2006
(TP06) [8], where the formulation of the intracellular calcium handling was modified by introducing a subspace to
describe the calcium induced calcium release. Fig. 7.3 shows a detailed schematic mode of the TP06 model.
The major innovation of the TP06 model with respect to its predecessor is the modification of the calcium handling
and the calcium current through the L-type channels. The improved formulation of calcium handling includes a sub-
space (SS) between the sarcoplasmic reticulum (SR) and the cell membrane from which the release of calcium into the
cytoplasm is performed. Meanwhile, the release of calcium from the SR to SS is carried out through a mechanism con-
trolled by the Ryanodine receptors, whose dynamic is governed by a Markov chain of four states (see Fig. 7.3). How-
ever, in the implementation of the TP06, the dimensionality of the Markov chain is reduced to two states in order to
reduce the computational cost of the model. With the introduction of the SS, the flow of calcium through the L-type
2+
channels injects calcium into the SS, this being inactivated now by the concentration of calcium in the SS [Ca ] SS
2+
instead of [Ca ] i . In addition, the new formulation includes two gates of inactivation depending on the potential,
a slow and a fast one to accommodate additional experimental observations.
In this chapter, the TP06 model has been used to study the electric activity of the human heart in normal and path-
ological conditions, considering the transmural heterogeneity (different proportions of cells of the epicardium, the
endocardium, and the midmyocardium). Its response has also been featured under conditions of ischemia (hyperka-
lemia, acidosis, and hypoxia). To study the effect of hypoxia, a modification of the I KATP model of Ferrero [48] has been
done. Finally, this modified model has been used to study the electrical response of the heart under conditions of acute
FIG. 7.3 Ten Tusscher model 2006 [8].
I. BIOMECHANICS