Page 131 - Advances in Biomechanics and Tissue Regeneration
P. 131
7.3 VULNERABILITY IN REGIONALLY ISCHEMIC HUMAN HEART: EFFECT OF THE EXTRACELLULAR POTASSIUM CONCENTRATION 127
From a mathematical and computational point of view, the electrophysiology problem is the coupled solution of a
linear partial differential equation, describing electric conduction, with a nonlinear stiff system of ODEs describing the
cellular ionic currents that lead to a nonlinear reaction-diffusion problem. An efficient way of solving Eqs. (7.43)–(7.45)
is by applying the Strang-based operator splitting scheme [49] in combination with a trapezoidal family method for
time integrations, in conjunction with the FEM for the spatial discretization [59].
7.3.2 Model of Acute Ischemia
Simulation of the ischemic heart requires an accurate description of the organ that incorporates both its muscular
structure and heterogeneity (described in the following sections), and an appropriate model of its electrophysiology.
This section is dedicated to the characterization of the mathematical model of AP used to perform the numerical sim-
ulations of the regional acute ischemic heart. All simulations were performed with a modified version of the ten
Tusscher and Panfilov (TP06) model of AP [8].
7.3.2.1 Action Potential Model Under Ischemic Conditions
One of the most important aspects in simulating the ischemic heart is to incorporate ATP-sensitive potassium cur-
rent I KATP , a dormant depolarization current under physiological conditions that is activated under ischemic condi-
tions [48]. K ATP ion channels have been investigated in different regions of the heart, that is, the atria and ventricle and
the sinoatrial (SA) and atrioventricular (AV) nodes, on different species [60–63]. However, very little experimental data
regarding the I KATP current for different tissue layers within the ventricle are available. Furukawa et al. [60] have char-
acterized K ATP channels in isolated endocardial and epicardial cells of cats. Experiments by Furukawa et al. suggest
that the open probability of K ATP channels reduces with the intracellular ATP concentration, [ATP] i , for both cell types.
However, the [ATP] i concentration responsible for a 50% block of K ATP channels is approximately four times less for
endocardial cells than for epicardial cells. Similar observations have been made by Nichols et al. [61] and Venkatesh
et al. [62] in the epicardial cells of guinea pigs, and by Light et al. [63] in rabbits.
A modified version of the ten Tusscher cardiac AP model [8] was used in the simulations. The model describes the
principal ionic currents through the cardiac cell membrane with a high degree of electrophysiological detail for the
+
three types of cardiac cells. The basic model was completed with the formulation of the ATP-sensitive K current
(I KATP ) described by Ferrero et al. [48].
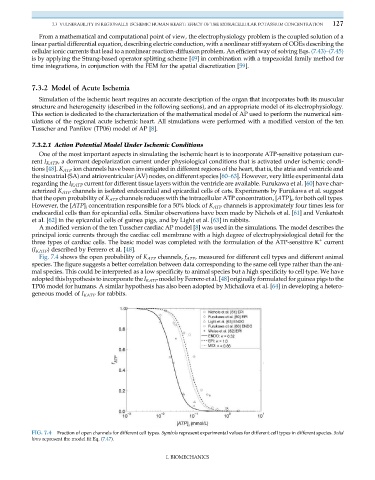
Fig. 7.4 shows the open probability of K ATP channels, f ATP , measured for different cell types and different animal
species. The figure suggests a better correlation between data corresponding to the same cell type rather than the ani-
mal species. This could be interpreted as a low specificity to animal species but a high specificity to cell type. We have
adopted this hypothesis to incorporate the I KATP model by Ferrero et al. [48] originally formulated for guinea pigs to the
TP06 model for humans. A similar hypothesis has also been adopted by Michailova et al. [64] in developing a hetero-
geneous model of I KATP for rabbits.
FIG. 7.4 Fraction of open channels for different cell types. Symbols represent experimental values for different cell types in different species. Solid
lines represent the model fit Eq. (7.47).
I. BIOMECHANICS