Page 407 - Advances in Eco-Fuels for a Sustainable Environment
P. 407
Engine modification for alternative fuels usage in diesel engine 361
13.2.4 Methyl ester characterization
Ester formation and determination of biodiesel conversion percentage can be
1
measured through proton nuclear magnetic resonance ( H NMR, Make-
1
BRUKER, Model-400MHz). In H NMR analysis, tetramethylsilane used as
1
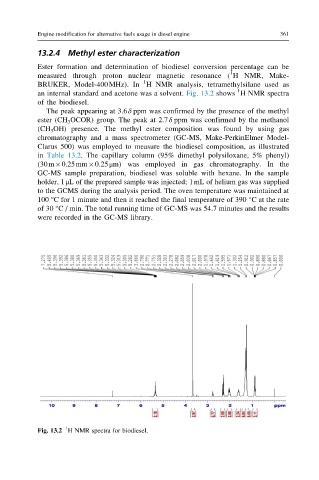
an internal standard and acetone was a solvent. Fig. 13.2 shows HNMR spectra
of the biodiesel.
The peak appearing at 3.6δ ppm was confirmed by the presence of the methyl
ester (CH 3 OCOR) group. The peak at 2.7δ ppm was confirmed by the methanol
(CH 3 OH) presence. The methyl ester composition was found by using gas
chromatography and a mass spectrometer (GC-MS, Make-PerkinElmer Model-
Clarus 500) was employed to measure the biodiesel composition, as illustrated
in Table 13.2. The capillary column (95% dimethyl polysiloxane, 5% phenyl)
(30m 0.25mm 0.25μm) was employed in gas chromatography. In the
GC-MS sample preparation, biodiesel was soluble with hexane. In the sample
holder, 1μL of the prepared sample was injected; 1mL of helium gas was supplied
to the GCMS during the analysis period. The oven temperature was maintained at
100 °C for 1 minute and then it reached the final temperature of 390 °Catthe rate
of 30 °C/min. Thetotal runningtimeofGC-MS was54.7minutesandthe results
were recorded in the GC-MS library.
1
Fig. 13.2 H NMR spectra for biodiesel.