Page 288 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 288
9.3 SO 2 Capture by Furnace Sorbent Injection 265
Sorbent injection Humidification Clean air
Particulate
Air control devices
heater
CaCO 3 or Boiler
Ca(OH) 2
Stack
Disposal
Reactivation
Product
Optional Recycle
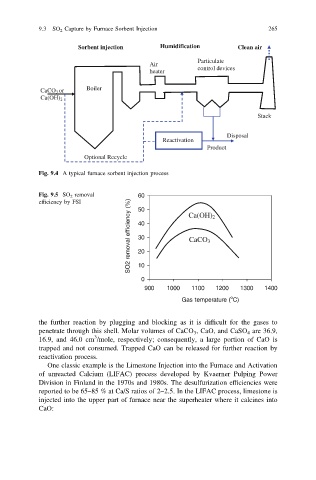
Fig. 9.4 A typical furnace sorbent injection process
Fig. 9.5 SO 2 removal 60
SO2 removal efficiency (%) 40 CaCO 3
efficiency by FSI 50 Ca(OH) 2
30
20
10
0
900 1000 1100 1200 1300 1400
o
Gas temperature ( C)
the further reaction by plugging and blocking as it is difficult for the gases to
penetrate through this shell. Molar volumes of CaCO 3 , CaO, and CaSO 4 are 36.9,
3
16.9, and 46.0 cm /mole, respectively; consequently, a large portion of CaO is
trapped and not consumed. Trapped CaO can be released for further reaction by
reactivation process.
One classic example is the Limestone Injection into the Furnace and Activation
of unreacted Calcium (LIFAC) process developed by Kvaerner Pulping Power
Division in Finland in the 1970s and 1980s. The desulfurization efficiencies were
reported to be 65–85 % at Ca/S ratios of 2–2.5. In the LIFAC process, limestone is
injected into the upper part of furnace near the superheater where it calcines into
CaO: