Page 290 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 290
9.3 SO 2 Capture by Furnace Sorbent Injection 267
9.3.2 SO 2 Capture in Fluidized Bed Combustion
As mentioned above in FBC SO 2 sorbent can be mixed with other bed materials for
desulfurization, since the temperature in chamber is low, CaSO 4 formed remains a
stable compound. In addition to relatively low-NO x emissions due to low com-
bustion temperature, FBC can also capture H 2 S, if any, forming CaS, which can be
oxidized to CaSO 4 , before it is oxidized into SO 2 .
In addition to Reactions (9.6) and (9.7), direct sulfation may also take place.
Similar to fixed bed combustion, the formation of CaSO 4 in FBC also results in the
partially used sorbent, and the reactivation of spent sorbent will give higher effi-
ciency at lower cost of sorbent.
1
CaCO 3 þ = 2O 2 þ SO 2 ! CaSO 4 þ CO 2 ð9:10Þ
This reaction is relatively slow because calcined limestone, CaO, is more
reactive than uncalcined limestone, CaCO 3 . In reality, the calcareous materials
mined have different purity and chemical compositions, leading to large differences
in SO 2 removal efficiencies, even though they are tested at identical conditions.
Desulfurization by injection of sorbent into an atmospheric FBC performs the
best at 800–850 °C, which is more sensitive for BFBC than CFBC. This maximum
temperature was mainly related to the stability of the CaSO 4 . It becomes unstable at
a temperature above 850 °C, when part of the desulfurization product, CaSO 4 , react
with CO and/or H 2 to produce CaS, CaO or CaCO 3 , depending on temperature, and
partial pressures of the reactants, via a complex chemistry that involves complicated
step reactions [18, 21], and they are not listed herein.
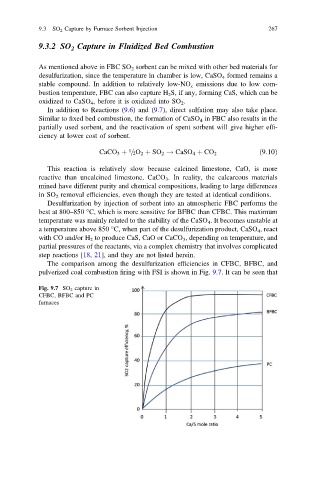
The comparison among the desulfurization efficiencies in CFBC, BFBC, and
pulverized coal combustion firing with FSI is shown in Fig. 9.7. It can be seen that
Fig. 9.7 SO 2 capture in
CFBC, BFBC and PC
furnaces