Page 295 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 295
272 9 In-combustion Air Emission Control
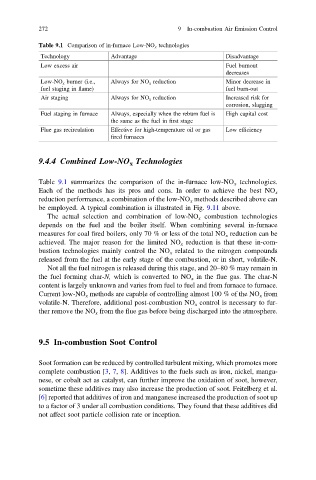
Table 9.1 Comparison of in-furnace Low-NO x technologies
Technology Advantage Disadvantage
Low excess air Fuel burnout
decreases
Low-NO x burner (i.e., Always for NO x reduction Minor decrease in
fuel staging in flame) fuel burn-out
Air staging Always for NO x reduction Increased risk for
corrosion, slagging
Fuel staging in furnace Always, especially when the reburn fuel is High capital cost
the same as the fuel in first stage
Flue gas recirculation Effective for high-temperature oil or gas Low efficiency
fired furnaces
9.4.4 Combined Low-NO Technologies
x
Table 9.1 summarizes the comparison of the in-furnace low-NO x technologies.
Each of the methods has its pros and cons. In order to achieve the best NO x
reduction performance, a combination of the low-NO x methods described above can
be employed. A typical combination is illustrated in Fig. 9.11 above.
The actual selection and combination of low-NO x combustion technologies
depends on the fuel and the boiler itself. When combining several in-furnace
measures for coal fired boilers, only 70 % or less of the total NO x reduction can be
achieved. The major reason for the limited NO x reduction is that these in-com-
bustion technologies mainly control the NO x related to the nitrogen compounds
released from the fuel at the early stage of the combustion, or in short, volatile-N.
Not all the fuel nitrogen is released during this stage, and 20–80 % may remain in
the fuel forming char-N, which is converted to NO x in the flue gas. The char-N
content is largely unknown and varies from fuel to fuel and from furnace to furnace.
Current low-NO x methods are capable of controlling almost 100 % of the NO x from
volatile-N. Therefore, additional post-combustion NO x control is necessary to fur-
ther remove the NO x from the flue gas before being discharged into the atmosphere.
9.5 In-combustion Soot Control
Soot formation can be reduced by controlled turbulent mixing, which promotes more
complete combustion [3, 7, 8]. Additives to the fuels such as iron, nickel, manga-
nese, or cobalt act as catalyst, can further improve the oxidation of soot, however,
sometime these additives may also increase the production of soot. Feitelberg et al.
[6] reported that additives of iron and manganese increased the production of soot up
to a factor of 3 under all combustion conditions. They found that these additives did
not affect soot particle collision rate or inception.