Page 462 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 462
14.4 Indoor Air Cleaning Model 443
Example 14.4: Transient indoor air pollutant concentration
3
Consider a commercial kitchen with a volume of 80 m where natural gas is used as
a cooking fuel. Assume that after cooking the indoor fume concentration is
3 3
1,000 µg/m . The HVAC system works with a fresh air flow rate of 10 m /s and an
internal recirculating air flow rate of 1/3 of the fresh air flow rate. Ignore the
outdoor and indoor source other than cooking. The intake and recirculating filter
3
efficiencies are both 90 % and the internal air cleaner has a flow rate of 1 m /s and
an efficiency of 95 %. Plot the indoor fume concentration over time.
Solution
The problem is simplified by ignoring the outdoor and indoor sources other than
cooking. After cooking, _ s = 0, and c 0 ¼ 0 in Eq. (14.23). This simplification leads to
Q o c o 1 g Þ þ _ s
ð
o
c i1 ¼ ¼ 0
Q o Q r 1 gð r Þ þ Q i g i
Then, Eq. (14.22) becomes
t
c i ðtÞ¼ c i0 exp
s
where the time constant is calculated using Eq. (14.24):
V 800
s ¼ ¼ 10 ¼ 75:35ðsÞ
Q o Q r 1 gð Þ þ Q i g 10 ð 1 0:9Þ þ 1 0:95
r i 3
Then we have
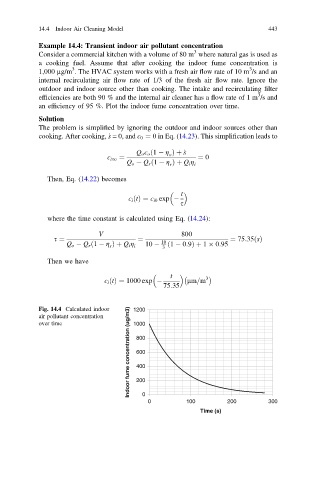
t 3
c i ðtÞ¼ 1000 exp lm=m
75:35
Indoor fume concentration (ug/m3) 600
Fig. 14.4 Calculated indoor 1200
air pollutant concentration
over time 1000
800
400
200
0
0 100 200 300
Time (s)