Page 39 - An Introduction to Microelectromechanical Systems Engineering
P. 39
18 Materials for MEMS
Beam structures made of polycrystalline or amorphous silicon that have not been
subjected to a careful stress annealing step can curl under the effect of intrinsic
stress.
Silicon is a very good thermal conductor with a thermal conductivity greater than
that of many metals and approximately 100 times larger than that of glass. In com-
plex integrated systems, the silicon substrate can be used as an efficient heat sink.
This feature will be revisited when we review thermal-based sensors and actuators.
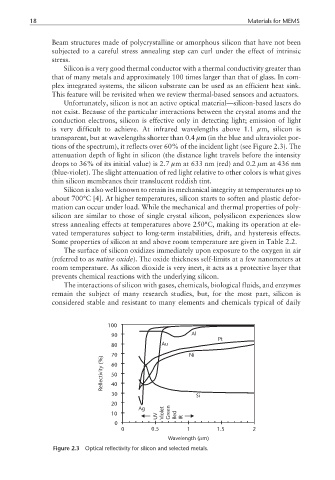
Unfortunately, silicon is not an active optical material—silicon-based lasers do
not exist. Because of the particular interactions between the crystal atoms and the
conduction electrons, silicon is effective only in detecting light; emission of light
is very difficult to achieve. At infrared wavelengths above 1.1 µm, silicon is
transparent, but at wavelengths shorter than 0.4 µm (in the blue and ultraviolet por-
tions of the spectrum), it reflects over 60% of the incident light (see Figure 2.3). The
attenuation depth of light in silicon (the distance light travels before the intensity
drops to 36% of its initial value) is 2.7 µm at 633 nm (red) and 0.2 µm at 436 nm
(blue-violet). The slight attenuation of red light relative to other colors is what gives
thin silicon membranes their translucent reddish tint.
Silicon is also well known to retain its mechanical integrity at temperatures up to
about 700°C [4]. At higher temperatures, silicon starts to soften and plastic defor-
mation can occur under load. While the mechanical and thermal properties of poly-
silicon are similar to those of single crystal silicon, polysilicon experiences slow
stress annealing effects at temperatures above 250°C, making its operation at ele-
vated temperatures subject to long-term instabilities, drift, and hysteresis effects.
Some properties of silicon at and above room temperature are given in Table 2.2.
The surface of silicon oxidizes immediately upon exposure to the oxygen in air
(referred to as native oxide). The oxide thickness self-limits at a few nanometers at
room temperature. As silicon dioxide is very inert, it acts as a protective layer that
prevents chemical reactions with the underlying silicon.
The interactions of silicon with gases, chemicals, biological fluids, and enzymes
remain the subject of many research studies, but, for the most part, silicon is
considered stable and resistant to many elements and chemicals typical of daily
100
90 Al
Pt
80 Au
70 Ni
(%) 60
Reflectivity 50
40
30 Si
20
Ag
10 Violet Green Red
UV IR
0
0 0.5 1 1.5 2
µ
Wavelength ( m)
Figure 2.3 Optical reflectivity for silicon and selected metals.