Page 395 - Analysis, Synthesis and Design of Chemical Processes, Third Edition
P. 395
In the preceding section, a method for breaking binary azeotropes was mentioned that involved adding a
third component to break the azeotrope. A key question is how to pick the added component. One answer
is to pick an intermediate-boiling component that does not create a new azeotrope and has a residue curve
map without any boundaries like Figure 12.9(c). The distillation column sequence and the representation
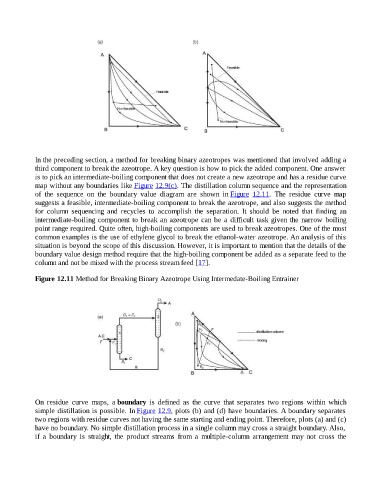
of the sequence on the boundary value diagram are shown in Figure 12.11. The residue curve map
suggests a feasible, intermediate-boiling component to break the azeotrope, and also suggests the method
for column sequencing and recycles to accomplish the separation. It should be noted that finding an
intermediate-boiling component to break an azeotrope can be a difficult task given the narrow boiling
point range required. Quite often, high-boiling components are used to break azeotropes. One of the most
common examples is the use of ethylene glycol to break the ethanol-water azeotrope. An analysis of this
situation is beyond the scope of this discussion. However, it is important to mention that the details of the
boundary value design method require that the high-boiling component be added as a separate feed to the
column and not be mixed with the process stream feed [17].
Figure 12.11 Method for Breaking Binary Azeotrope Using Intermedate-Boiling Entrainer
On residue curve maps, a boundary is defined as the curve that separates two regions within which
simple distillation is possible. In Figure 12.9, plots (b) and (d) have boundaries. A boundary separates
two regions with residue curves not having the same starting and ending point. Therefore, plots (a) and (c)
have no boundary. No simple distillation process in a single column may cross a straight boundary. Also,
if a boundary is straight, the product streams from a multiple-column arrangement may not cross the