Page 122 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 122
4-5 WORKING ELECTRODES 107
4-4). The autosampler can accommodate over 100 samples, as well as relevant
standard solutions. Such coupling can also address the preliminary stages of sample
preparation (as dictated by the nature of the sample). The role of computers in
electroanalytical measurements and in the development of ``smarter'' analyzers has
been reviewed by Bond (7) and He et al. (8).
The nature of electrochemical instruments makes them very attractive for
decentralized testing. For example, compact, battery-operated voltammetric analy-
zers, developed for on-site measurements of metals (9,10), readily address the
growing needs for ®eld-based environmental studies. Similarly, portable (hand-held)
instruments are being designed for decentralized clinical testing (11).
4-5 WORKING ELECTRODES
The performance of the voltammetric procedure is strongly in¯uenced by the
material of the working electrode. The working electrode should provide high
signal-to-noise characteristics, as well as a reproducible response. Thus, its selection
depends primarily on two factors: the redox behavior of the target analyte and the
background current over the potential region required for the measurement. Other
considerations include the potential window, electrical conductivity, surface repro-
ducibility, mechanical properties, cost, availability, and toxicity. A range of materials
have found application as working electrodes for electroanalysis. The most popular
are those involving mercury, carbon, or noble metals (particularly platinum and
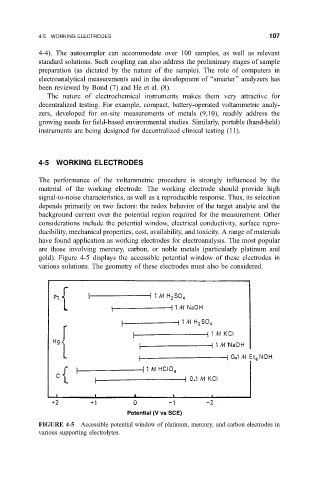
gold). Figure 4-5 displays the accessible potential window of these electrodes in
various solutions. The geometry of these electrodes must also be considered.
FIGURE 4-5 Accessible potential window of platinum, mercury, and carbon electrodes in
various supporting electrolytes.