Page 123 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 123
108 PRACTICAL CONSIDERATIONS
4-5.1 Mercury Electrodes
Mercury is a very attractive choice of electrode material because it has a high
hydrogen overvoltage that greatly extends the cathodic potential window (compared
to solid electrode materials) and possesses a highly reproducible, readily renewable,
and smooth surface. In electrochemical terms, its roughness factor equals unity (i.e.,
identical geometrical and actual surface areas). Disadvantages of the use of mercury
are its limited anodic range (due to the oxidation of mercury) and its toxicity.
There are several types of mercury electrodes. Of these, the dropping mercury
electrode (DME), the hanging mercury drop electrode (HMDE), and mercury ®lm
electrode (MFE) are the most frequently used.
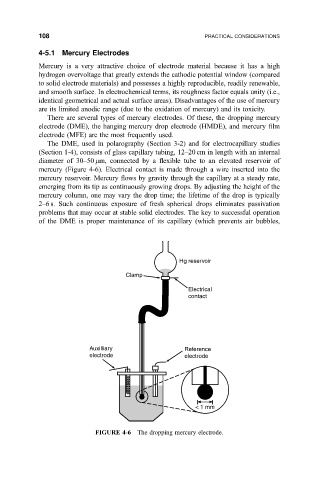
The DME, used in polarography (Section 3-2) and for electrocapillary studies
(Section 1-4), consists of glass capillary tubing, 12±20 cm in length with an internal
diameter of 30±50 mm, connected by a ¯exible tube to an elevated reservoir of
mercury (Figure 4-6). Electrical contact is made through a wire inserted into the
mercury reservoir. Mercury ¯ows by gravity through the capillary at a steady rate,
emerging from its tip as continuously growing drops. By adjusting the height of the
mercury column, one may vary the drop time; the lifetime of the drop is typically
2±6 s. Such continuous exposure of fresh spherical drops eliminates passivation
problems that may occur at stable solid electrodes. The key to successful operation
of the DME is proper maintenance of its capillary (which prevents air bubbles,
Hg reservoir
Clamp
Electrical
contact
Auxilliary Reference
electrode electrode
< 1 mm
FIGURE 4-6 The dropping mercury electrode.