Page 142 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 142
4-5 WORKING ELECTRODES 127
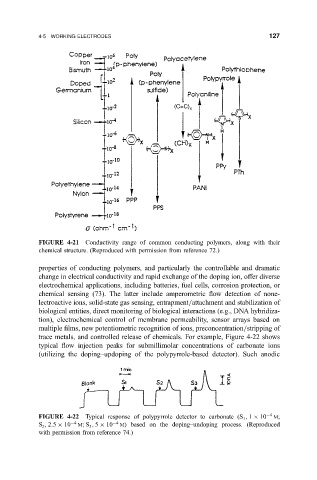
FIGURE 4-21 Conductivity range of common conducting polymers, along with their
chemical structure. (Reproduced with permission from reference 72.)
properties of conducting polymers, and particularly the controllable and dramatic
change in electrical conductivity and rapid exchange of the doping ion, offer diverse
electrochemical applications, including batteries, fuel cells, corrosion protection, or
chemical sensing (73). The latter include amperometric ¯ow detection of none-
lectroactive ions, solid-state gas sensing, entrapment=attachment and stabilization of
biological entities, direct monitoring of biological interactions (e.g., DNA hybridiza-
tion), electrochemical control of membrane permeability, sensor arrays based on
multiple ®lms, new potentiometric recognition of ions, preconcentration=stripping of
trace metals, and controlled release of chemicals. For example, Figure 4-22 shows
typical ¯ow injection peaks for submillimolar concentrations of carbonate ions
(utilizing the doping±updoping of the polypyrrole-based detector). Such anodic
FIGURE 4-22 Typical response of polypyrrole detector to carbonate (S ; 1 10 4 M;
1
S ; 2:5 10 4 M; S ; 5 10 4 M) based on the doping±undoping process. (Reproduced
3
2
with permission from reference 74.)