Page 141 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 141
126 PRACTICAL CONSIDERATIONS
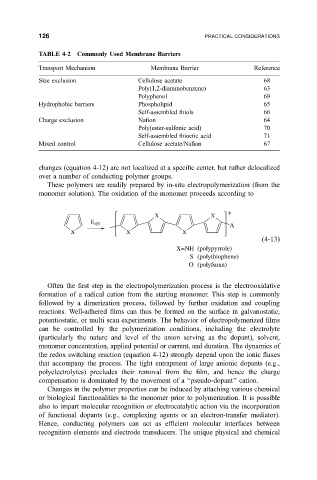
TABLE 4-2 Commonly Used Membrane Barriers
Transport Mechanism Membrane Barrier Reference
Size exclusion Cellulose acetate 68
Poly(1,2-diaminobenzene) 63
Polyphenol 69
Hydrophobic barriers Phospholipid 65
Self-assembled thiols 66
Charge exclusion Na®on 64
Poly(ester-sulfonic acid) 70
Self-assembled thioctic acid 71
Mixed control Cellulose acetate/Na®on 67
changes (equation 4-12) are not localized at a speci®c center, but rather delocalized
over a number of conducting polymer groups.
These polymers are readily prepared by in-situ electropolymerization (from the
monomer solution). The oxidation of the monomer proceeds according to
+
X X
E app
A
X X X
4-13
X=NH (polypyrrole)
S (polythiophene)
O (polyfuran)
Often the ®rst step in the electropolymerization process is the electrooxidative
formation of a radical cation from the starting monomer. This step is commonly
followed by a dimerization process, followed by further oxidation and coupling
reactions. Well-adhered ®lms can thus be formed on the surface in galvanostatic,
potentiostatic, or multi scan experiments. The behavior of electropolymerized ®lms
can be controlled by the polymerization conditions, including the electrolyte
(particularly the nature and level of the anion serving as the dopant), solvent,
monomer concentration, applied potential or current, and duration. The dynamics of
the redox switching reaction (equation 4-12) strongly depend upon the ionic ¯uxes
that accompany the process. The tight entrapment of large anionic dopants (e.g.,
polyelectrolytes) precludes their removal from the ®lm, and hence the charge
compensation is dominated by the movement of a ``pseudo-dopant'' cation.
Changes in the polymer properties can be induced by attaching various chemical
or biological functionalities to the monomer prior to polymerization. It is possible
also to impart molecular recognition or electrocatalytic action via the incorporation
of functional dopants (e.g., complexing agents or an electron-transfer mediator).
Hence, conducting polymers can act as ef®cient molecular interfaces between
recognition elements and electrode transducers. The unique physical and chemical