Page 138 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 138
4-5 WORKING ELECTRODES 123
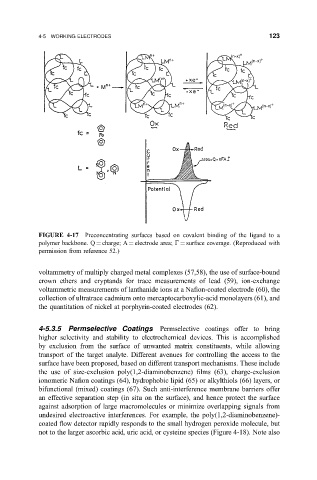
FIGURE 4-17 Preconcentrating surfaces based on covalent binding of the ligand to a
polymer backbone. Q charge; A electrode area; G surface coverage. (Reproduced with
permission from reference 52.)
voltammetry of multiply charged metal complexes (57,58), the use of surface-bound
crown ethers and cryptands for trace measurements of lead (59), ion-exchange
voltammetric measurements of lanthanide ions at a Na®on-coated electrode (60), the
collection of ultratrace cadmium onto mercaptocarboxylic-acid monolayers (61), and
the quantitation of nickel at porphyrin-coated electrodes (62).
4-5.3.5 Permselective Coatings Permselective coatings offer to bring
higher selectivity and stability to electrochemical devices. This is accomplished
by exclusion from the surface of unwanted matrix constituents, while allowing
transport of the target analyte. Different avenues for controlling the access to the
surface have been proposed, based on different transport mechanisms. These include
the use of size-exclusion poly(1,2-diaminobenzene) ®lms (63), charge-exclusion
ionomeric Na®on coatings (64), hydrophobic lipid (65) or alkylthiols (66) layers, or
bifunctional (mixed) coatings (67). Such anti-interference membrane barriers offer
an effective separation step (in situ on the surface), and hence protect the surface
against adsorption of large macromolecules or minimize overlapping signals from
undesired electroactive interferences. For example, the poly(1,2-diaminobenzene)-
coated ¯ow detector rapidly responds to the small hydrogen peroxide molecule, but
not to the larger ascorbic acid, uric acid, or cysteine species (Figure 4-18). Note also