Page 134 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 134
4-5 WORKING ELECTRODES 119
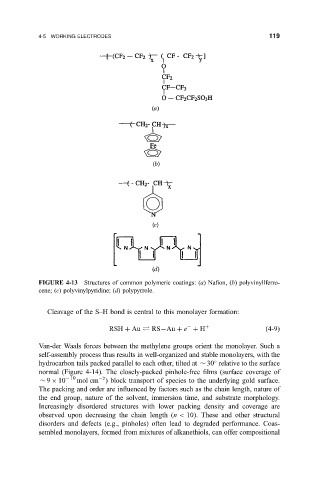
FIGURE 4-13 Structures of common polymeric coatings: (a) Na®on, (b) polyvinyllferro-
cene; (c) polyvinylpyridine; (d) polypyrrole.
Cleavage of the S±H bond is central to this monolayer formation:
RSH Au RS Au e H
4-9
Van-der Waals forces between the methylene groups orient the monolayer. Such a
self-assembly process thus results in well-organized and stable monolayers, with the
hydrocarbon tails packed parallel to each other, tilted at 30 relative to the surface
normal (Figure 4-14). The closely-packed pinhole-free ®lms (surface coverage of
2
9 10 10 mol cm ) block transport of species to the underlying gold surface.
The packing and order are in¯uenced by factors such as the chain length, nature of
the end group, nature of the solvent, immersion time, and substrate morphology.
Increasingly disordered structures with lower packing density and coverage are
observed upon decreasing the chain length (n < 10). These and other structural
disorders and defects (e.g., pinholes) often lead to degraded performance. Coas-
sembled monolayers, formed from mixtures of alkanethiols, can offer compositional