Page 132 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 132
4-5 WORKING ELECTRODES 117
4-5.2.3 Metal Electrodes While a wide choice of noble metals is available,
platinum and gold are the most widely used metallic electrodes. Such electrodes
offer a very favorable electron-transfer kinetics and a large anodic potential range. In
contrast, the low hydrogen overvoltage at these electrode limits the cathodic
potential window (to the 0.2 to 0.5 V region, depending upon the pH). More
problematic are the high background currents associated with the formation of
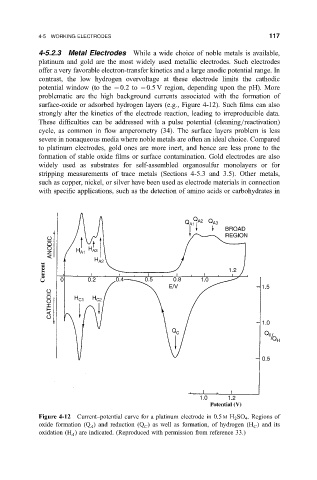
surface-oxide or adsorbed hydrogen layers (e.g., Figure 4-12). Such ®lms can also
strongly alter the kinetics of the electrode reaction, leading to irreproducible data.
These dif®culties can be addressed with a pulse potential (cleaning=reactivation)
cycle, as common in ¯ow amperometry (34). The surface layers problem is less
severe in nonaqueous media where noble metals are often an ideal choice. Compared
to platinum electrodes, gold ones are more inert, and hence are less prone to the
formation of stable oxide ®lms or surface contamination. Gold electrodes are also
widely used as substrates for self-assembled organosulfur monolayers or for
stripping measurements of trace metals (Sections 4-5.3 and 3.5). Other metals,
such as copper, nickel, or silver have been used as electrode materials in connection
with speci®c applications, such as the detection of amino acids or carbohydrates in
Figure 4-12 Current±potential curve for a platinum electrode in 0.5 M H 2 SO 4 . Regions of
oxide formation (Q ) and reduction (Q ) as well as formation, of hydrogen (H ) and its
A
C
C
oxidation (H ) are indicated. (Reproduced with permission from reference 33.)
A