Page 136 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 136
4-5 WORKING ELECTRODES 121
(e.g., porosity), thermal stability, and mechanical rigidity. Sol-gel-derived composite
electrodes have also been prepared by dispersing carbon or gold powders in the
initial sol-gel mixture (43,44).
4-5.3.3 Electrocatalytic Modi®ed Electrodes Often the desired redox
reaction at the bare electrode involves slow electron-transfer kinetics and therefore
occurs at an appreciable rate only at potentials substantially higher than its
thermodynamic redox potential. Such reactions can be catalyzed by attaching to
the surface a suitable electron transfer mediator (45,46). Knowledge of homoge-
neous solution kinetics is often used to select the surface-bound catalyst. The
function of the mediator is to facilitate the charge transfer between the analyte and
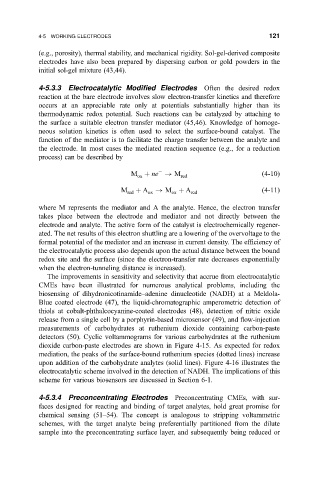
the electrode. In most cases the mediated reaction sequence (e.g., for a reduction
process) can be described by
M ne ! M
4-10
ox red
M red A ox ! M A red
4-11
ox
where Mrepresents the mediator and A the analyte. Hence, the electron transfer
takes place between the electrode and mediator and not directly between the
electrode and analyte. The active form of the catalyst is electrochemically regener-
ated. The net results of this electron shuttling are a lowering of the overvoltage to the
formal potential of the mediator and an increase in current density. The ef®ciency of
the electrocatalytic process also depends upon the actual distance between the bound
redox site and the surface (since the electron-transfer rate decreases exponentially
when the electron-tunneling distance is increased).
The improvements in sensitivity and selectivity that accrue from electrocatalytic
CMEs have been illustrated for numerous analytical problems, including the
biosensing of dihydronicotinamide±adenine dinucleotide (NADH) at a Meldola-
Blue coated electrode (47), the liquid-chromatographic amperometric detection of
thiols at cobalt-phthalcocyanine-coated electrodes (48), detection of nitric oxide
release from a single cell by a porphyrin-based microsensor (49), and ¯ow-injection
measurements of carbohydrates at ruthenium dioxide containing carbon-paste
detectors (50). Cyclic voltammograms for various carbohydrates at the ruthenium
dioxide carbon-paste electrodes are shown in Figure 4-15. As expected for redox
mediation, the peaks of the surface-bound ruthenium species (dotted lines) increase
upon addition of the carbohydrate analytes (solid lines). Figure 4-16 illustrates the
electrocatalytic scheme involved in the detection of NADH. The implications of this
scheme for various biosensors are discussed in Section 6-1.
4-5.3.4 Preconcentrating Electrodes Preconcentrating CMEs, with sur-
faces designed for reacting and binding of target analytes, hold great promise for
chemical sensing (51±54). The concept is analogous to stripping voltammetric
schemes, with the target analyte being preferentially partitioned from the dilute
sample into the preconcentrating surface layer, and subsequently being reduced or