Page 137 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 137
122 PRACTICAL CONSIDERATIONS
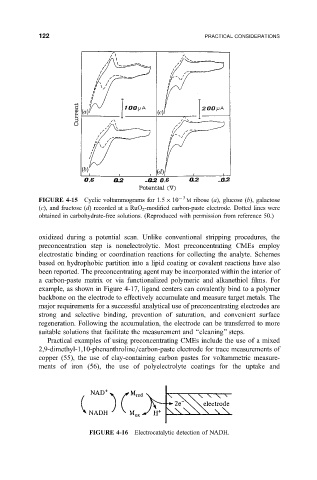
FIGURE 4-15 Cyclic voltammograms for 1.5 10 3 M ribose (a), glucose (b), galactose
(c), and fructose (d) recorded at a RuO 2 -modi®ed carbon-paste electrode. Dotted lines were
obtained in carbohydrate-free solutions. (Reproduced with permission from reference 50.)
oxidized during a potential scan. Unlike conventional stripping procedures, the
preconcentration step is nonelectrolytic. Most preconcentrating CMEs employ
electrostatic binding or coordination reactions for collecting the analyte. Schemes
based on hydrophobic partition into a lipid coating or covalent reactions have also
been reported. The preconcentrating agent may be incorporated within the interior of
a carbon-paste matrix or via functionalized polymeric and alkanethiol ®lms. For
example, as shown in Figure 4-17, ligand centers can covalently bind to a polymer
backbone on the electrode to effectively accumulate and measure target metals. The
major requirements for a successful analytical use of preconcentrating electrodes are
strong and selective binding, prevention of saturation, and convenient surface
regeneration. Following the accumulation, the electrode can be transferred to more
suitable solutions that facilitate the measurement and ``cleaning'' steps.
Practical examples of using preconcentrating CMEs include the use of a mixed
2,9-dimethyl-1,10-phenanthroline=carbon-paste electrode for trace measurements of
copper (55), the use of clay-containing carbon pastes for voltammetric measure-
ments of iron (56), the use of polyelectrolyte coatings for the uptake and
FIGURE 4-16 Electrocatalytic detection of NADH.