Page 63 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 63
Applied Process Design for Chemical and Petrochemical Plants
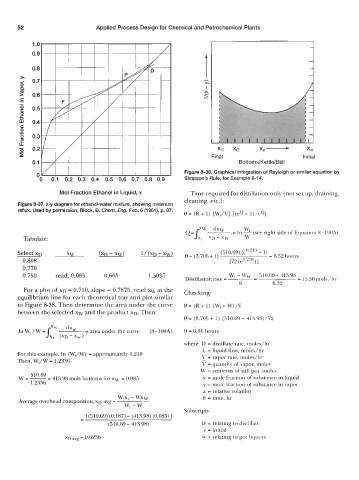
xx -
Xi1 Xi2 XI0
Final Initial
Bottoms/Kettle/Still
Figure 8-38. Graphical integration of Rayleigh or similar equation by
Simpson's Rule, for Example 8-14.
Mol Fraction Ethanol in Liquid, x Time required for distillation only (not set up, draining,
cleaning, etc.):
Figure 8-37. x-y diagram for ethanol-water mixture, showing minimum
reflux. Used by permission, Block, B. Chern. Eng. Feb. 6 (1961), p. 87.
0 = (R+ 1) [Wi/V] [(eQ- l)/eQ]
Wi
Q=Jxw dxw , = In - (see right side of Equation 8 - 100A)
Tabulate: xi XD-XW w
Select XD xw (XD - XW) 1/ (XD - xw)
0.808
0.770
0.750 read, 0.085 0.665 1.5037 Wi - Ww 510.69 - 413.98
-
Distillation rate = - = 15.30 mols/hr
0 6.32
For a plot of XD = 0.750, slope = 0.7875, read xw at the Checking:
equilibrium line for each theoretical tray and plot similar
to Figure 8-38. Then determine the area under the curve
between the selected xw and the product XD. Then:
e = 6.31 hours
where D = distillate rate, moles/hr
L = liquid flow, moles/hr
For this example, In (Wi/W) = approximately 0.210 V = vapor rate, moles/hr
Then, Wi/W = 1.2336 -
V = quantity of vapor, moles
W = contents of still pot, moles
w=-- 510'69 - 413.98 mols bottoms for xw = 0.085 x = mole fraction of substance in liquid
1.2336 y = mole fraction of substance in vapor
a = relative volatility
wixi - wxw 0 = time, hr
Average overhead composition, XD avg =
wi -w
Subscripts
- [(510.69) (0.187) - (413.98) (0.085)l
-
(510.69 - 413.98) D = relating to distillate
i = initial
XD = 0.6236 w = relating to pot liquors