Page 65 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 65
54 Applied Process Design for Chemical and Petrochemical Plants
a = relative volatility
01 = time for filling distillate receiver, hr
02 = time for refluxed distillation, hr
Example 8-16: Binary Batch Differential Distillation
Dimethyl ether is to be separated from methanol. A
batch type operation is to be tried to see if an existing coil-
in-tank can be used. The pressure of the system will be
about 55 psia. How many total mols will remain in the bot-
toms when the bottoms liquid composition contains 0.5
mol percent dimethyl ether? What is the composition of
the total overhead collected?
Initialcharge At104"F
Vap.
Mol Press.
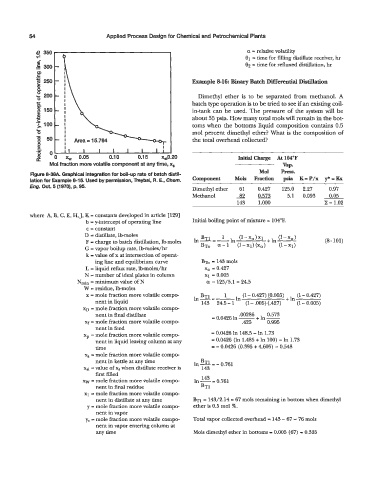
Figure 8-38A. Graphical integration for boil-up rate of batch distil-
lation for Example 8-15. Used by permission, Treybal, R. E., Chem. - Mols Fraction Psis K= p/x J* = &
Eng. Oct. 5 (1970), p. 95. Dimethyl ether 61 0.427 125.0 2.27 0.97
Methanol S2m 5.1 0.093 0.05
143 1.000 z = 1.02
where A, B, C, E, H, J, K = constants developed in article [ 1291
b = y-intercept of operating line Initial boiling point of mixture = 104°F.
c = constant
D = distillate, lb-moles
F = charge to batch distillation, lb-moles
G = vapor boilup rate, lbmoles/hr
k = value of x at intersection of operat-
ing line and equilibrium curve BTo = 143 mols
L = liquid reflux rate, lb-moles/hr x0 = 0.427
N = number of ideal plates in column XI = 0.005
Nmin = minimum value of N a = 123/5.1 = 24.5
W = residue, lb-moles
x = mole fraction more volatile compo- In-=- BT1 ln (1 - 0.427) (0.005) (1 - 0.427)
nent in liquid 143 24.5 - 1 (1 - .005) (.427) + In (1 - 0.005)
XD = mole fraction more volatile compo-
0.573
.00286
nent in final distillate = 0.0426 In -
+ln-
xf = mole fraction more volatile compo- .423 0.995
nent in feed
xp = mole fraction more volatile compo- = 0.0426 In 148.5 - In 1.73
nent in liquid leaving column at any = 0.0426 (In 1.485 + In 100) - In 1.73
time = - 0.0426 (0.395 + 4.605) - 0.548
x, = mole fraction more volatile compo-
nent in kettle at any time In - 0.761
-
BT1
xsi = value of x, when distillate receiver is 143 =
first filled
143
xw = mole fraction more volatile compo- In - 0.761
=
nent in final residue BT1
XI = mole fraction more volatile compo-
nent in distillate at any time = 143/2.14 = 67 mols remaining in bottom when dimethyl
y = mole fraction more volatile compo- ether is 0.5 mol %.
nent in vapor
y, = mole fraction more volatile compo- Total vapor collected overhead = 143 - 67 = 76 mols
nent in vapor entering column at
any time Mols dimethyl ether in bottoms = 0.005 (67) = 0.335