Page 69 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 69
58 Applied Process Design for Chemical and Petrochemical Plants
n = total system pressure (also see Equation 8 - 3) Any non-volatile material in the mixture will be left in the
still bottoms.
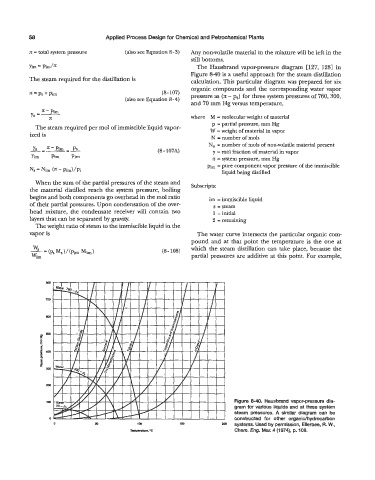
The Hausbrand vapor-pressure diagram [127, 1281 in
Figure &40 is a useful approach for the steam distillation
The steam required for the distillation is calculation. This particular diagram was prepared for six
organic compounds and the corresponding water vapor
* = Ps + Pirn (8-107) pressure as (n - p,) for three system pressures of 760,300,
(also see Equation 8-4)
and 70 mm Hg versus temperature,
* - Pim
Ys =- where M = molecular weight of material
ll
p = partial pressure, mm Hg
The steam required per mol of immiscible liquid vapor- W = weight of material in vapor
ized is N = number of mols
No =i number of mols of non-volatile material present
-=-=- X-Pim Ps (8- 107A) y = mol fraction of material in vapor
Ys
Yim F'im Pim x = system pressure, mm Hg
pim = pure component vapor pressure of the immiscible
liquid being distilled
When the sum of the partial pressures of the steam and Subscripts:
the material distilled reach the system pressure, boiling
begins and both components go overhead in the mol ratio im = immiscible liquid
of their partial pressures. Upon condensation of the over- s = steam
head mixture, the condensate receiver will contain two 1 =initial
layers that can be separated by gravity. 2 = remaining
The weight ratio of steam to the immiscible liquid in the
vapor is The water curve intersects the particular organic com-
pound and at that point the temperature is the one at
which the steam distillation can take place, because the
(8 - 108)
partial pressures are additive at this point. For example,
Figure 8-40. Hausbrand vapor-pressure dia-
gram for various liquids and at three system
steam pressures. A similar diagram can be
constructed for other organidhydrocarbon
systems. Used by permission, Ellerbee, R. W.,
Chem. Eng. Mar. 4 (1 974), p. 108.