Page 152 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 152
NITROGEN
132
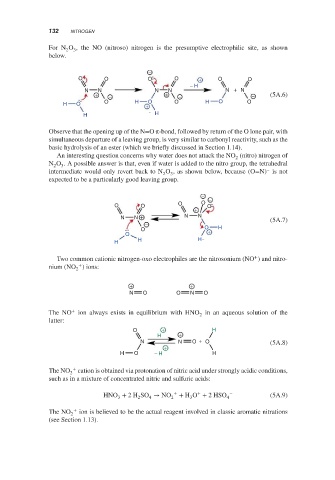
For N O , the NO (nitroso) nitrogen is the presumptive electrophilic site, as shown
2
3
below.
−
O O O O + O O
− H
N N N N N + N
+ − + − − (5A.6)
O H O O H O O
H O +
H H
Observe that the opening up of the N=O π-bond, followed by return of the O lone pair, with
simultaneous departure of a leaving group, is very similar to carbonyl reactivity, such as the
basic hydrolysis of an ester (which we briefly discussed in Section 1.14).
An interesting question concerns why water does not attack the NO (nitro) nitrogen of
2
N O . A possible answer is that, even if water is added to the nitro group, the tetrahedral
2 3
–
intermediate would only revert back to N O , as shown below, because (O=N) is not
2 3
expected to be a particularly good leaving group.
− −
O O O O O
+
N N + N N (5A.7)
−
O O + H
O
H H H
+
Two common cationic nitrogen-oxo electrophiles are the nitrosonium (NO ) and nitro-
+
nium (NO ) ions:
2
+ +
N O O N O
+
The NO ion always exists in equilibrium with HNO in an aqueous solution of the
2
latter:
O + H
H +
N N O + O (5A.8)
+
H O − H H
The NO 2 + cation is obtained via protonation of nitric acid under strongly acidic conditions,
such as in a mixture of concentrated nitric and sulfuric acids:
+ + −
HNO + 2H SO → NO 2 + H O + 2HSO 4 (5A.9)
3
2
3
4
+
The NO 2 ion is believed to be the actual reagent involved in classic aromatic nitrations
(see Section 1.13).