Page 156 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 156
NITROGEN
136
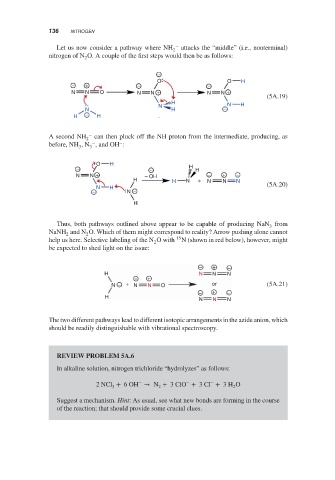
Let us now consider a pathway where NH 2 – attacks the “middle” (i.e., nonterminal)
nitrogen of N O. A couple of the first steps would then be as follows:
2
−
O O H
− + − −
N N O N N + N N +
(5A.19)
H
N N H
N H −
H − H
A second NH 2 – can then pluck off the NH proton from the intermediate, producing, as
–
–
before, NH ,N , and OH :
3
3
O H
− − H H
N N + − OH − + −
H H N + N N N
N H (5A.20)
− N −
H
Thus, both pathways outlined above appear to be capable of producing NaN from
3
NaNH and N O. Which of them might correspond to reality? Arrow pushing alone cannot
2
2
help us here. Selective labeling of the N O with 15 N (shown in red below), however, might
2
be expected to shed light on the issue:
− + −
H N N N
− +
N − + N N O or (5A.21)
− + −
H
N N N
The two different pathways lead to different isotopic arrangements in the azide anion, which
should be readily distinguishable with vibrational spectroscopy.
REVIEW PROBLEM 5A.6
In alkaline solution, nitrogen trichloride “hydrolyzes” as follows:
− − −
2NCl + 6OH → N + 3ClO + 3Cl + 3H O
2
3
2
Suggest a mechanism. Hint: As usual, see what new bonds are forming in the course
of the reaction; that should provide some crucial clues.