Page 147 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 147
4.12 SUMMARY 127
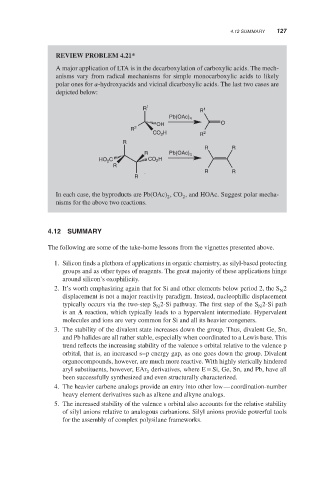
REVIEW PROBLEM 4.21*
A major application of LTA is in the decarboxylation of carboxylic acids. The mech-
anisms vary from radical mechanisms for simple monocarboxylic acids to likely
polar ones for -hydroxyacids and vicinal dicarboxylic acids. The last two cases are
depicted below:
R 1 1
R
Pb(OAc) 4
OH O
R 2
CO 2 H R 2
R
R R
R Pb(OAc) 4
HO 2 C CO 2 H
R
R R
R
In each case, the byproducts are Pb(OAc) ,CO , and HOAc. Suggest polar mecha-
2
2
nisms for the above two reactions.
4.12 SUMMARY
The following are some of the take-home lessons from the vignettes presented above.
1. Silicon finds a plethora of applications in organic chemistry, as silyl-based protecting
groups and as other types of reagents. The great majority of these applications hinge
around silicon’s oxophilicity.
2. It’s worth emphasizing again that for Si and other elements below period 2, the S 2
N
displacement is not a major reactivity paradigm. Instead, nucleophilic displacement
typically occurs via the two-step S 2-Si pathway. The first step of the S 2-Si path
N
N
is an A reaction, which typically leads to a hypervalent intermediate. Hypervalent
molecules and ions are very common for Si and all its heavier congeners.
3. The stability of the divalent state increases down the group. Thus, divalent Ge, Sn,
and Pb halides are all rather stable, especially when coordinated to a Lewis base. This
trend reflects the increasing stability of the valence s orbital relative to the valence p
orbital, that is, an increased s–p energy gap, as one goes down the group. Divalent
organocompounds, however, are much more reactive. With highly sterically hindered
aryl substituents, however, EAr derivatives, where E = Si, Ge, Sn, and Pb, have all
2
been successfully synthesized and even structurally characterized.
4. The heavier carbene analogs provide an entry into other low—coordination-number
heavy element derivatives such as alkene and alkyne analogs.
5. The increased stability of the valence s orbital also accounts for the relative stability
of silyl anions relative to analogous carbanions. Silyl anions provide powerful tools
for the assembly of complex polysilane frameworks.