Page 146 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 146
GROUP 14 ELEMENTS
126
OAc
AcO
Pb OAc − O
− OAc AcO R 4 R 3
O R 4 Pb + C + C
R 3 − HOAc 1 2
C C OAc R R (4.66)
O
R 1 O
R 2
H −
OAc
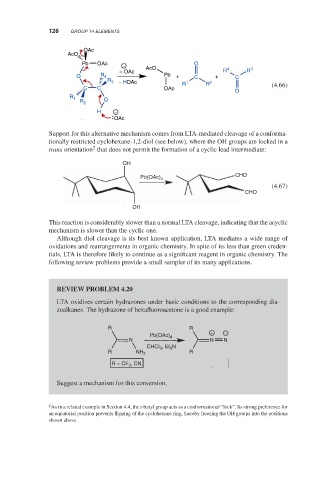
Support for this alternative mechanism comes from LTA-mediated cleavage of a conforma-
tionally restricted cyclohexane-1,2-diol (see below), where the OH groups are locked in a
2
trans orientation that does not permit the formation of a cyclic lead intermediate:
OH
CHO
Pb(OAc) 4
(4.67)
CHO
OH
This reaction is considerably slower than a normal LTA cleavage, indicating that the acyclic
mechanism is slower than the cyclic one.
Although diol cleavage is its best known application, LTA mediates a wide range of
oxidations and rearrangements in organic chemistry. In spite of its less than green creden-
tials, LTA is therefore likely to continue as a significant reagent in organic chemistry. The
following review problems provide a small sampler of its many applications.
REVIEW PROBLEM 4.20
LTA oxidizes certain hydrazones under basic conditions to the corresponding dia-
zoalkanes. The hydrazone of hexafluoroacetone is a good example:
R R
+ −
Pb(OAc) 4
N N N
CHCI 3 , Et 3 N
R NH 2 R
R = CF , CN
3
Suggest a mechanism for this conversion.
2 As in a related example in Section 4.4, the t-butyl group acts as a conformational “lock”. Its strong preference for
an equatorial position prevents flipping of the cyclohexane ring, thereby freezing the OH groups into the positions
shown above.