Page 145 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 145
4.11 GLYCOL CLEAVAGE BY LEAD TETRAACETATE 125
trivalent thallium, many tetravalent lead compounds are oxidizing. The functioning of the
now-discontinued antiknock agent tetraethyllead depends on its facile dissociation to lead
atoms and ethyl radicals, which results in smooth, controlled combustion of gasoline. In
organic chemistry, LTA in acetic acid is widely used as an oxidizing agent, particularly for
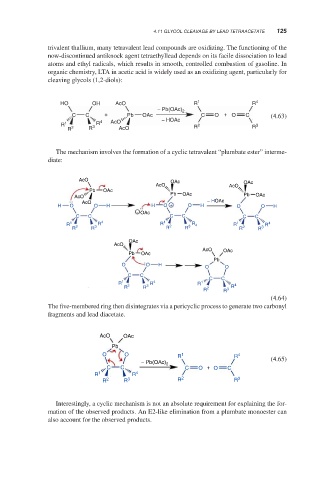
cleaving glycols (1,2-diols):
HO OH AcO R 1 R 4
− Pb(OAc) 2
C C + Pb OAc C O + O C (4.63)
R 1 3 R 4 AcO − HOAc R 2 R 3
R 2 R AcO
The mechanism involves the formation of a cyclic tetravalent “plumbate ester” interme-
diate:
AcO OAc OAc
AcO AcO
Pb OAc
Pb OAc Pb
AcO OAc
− HOAc
AcO
H O O H H O + O H O O H
− OAc
C C C C C C
R 1 R 4 R 1 2 3 R 4 R 1 R 4
R 2 R 3 R R R 2 R 3
OAc
AcO
AcO OAc
Pb OAc
Pb
O O H
O O
C C
C C
R 1 R 4 R 1 4
R 2 R 3 2 R
R R 3
(4.64)
The five-membered ring then disintegrates via a pericyclic process to generate two carbonyl
fragments and lead diacetate.
AcO OAc
Pb
O O R 1 R 4
− Pb(OAc) 2 (4.65)
C C C O + O C
R 1 R 4
R 2 R 3 R 2 R 3
Interestingly, a cyclic mechanism is not an absolute requirement for explaining the for-
mation of the observed products. An E2-like elimination from a plumbate monoester can
also account for the observed products.