Page 140 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 140
GROUP 14 ELEMENTS
120
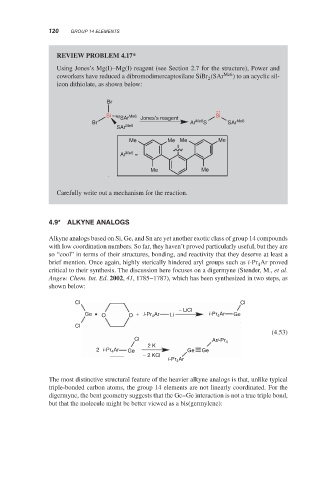
REVIEW PROBLEM 4.17*
Using Jones’s Mg(I)–Mg(I) reagent (see Section 2.7 for the structure), Power and
coworkers have reduced a dibromodimercaptosilane SiBr (SAr Me6 ) to an acyclic sil-
2
icon dithiolate, as shown below:
Br
Si Me6 Si
SAr Jones′s reagent
Br Ar Me6 S SAr Me6
SAr Me6
Me Me Me Me
Ar Me6 =
Me Me
Carefully write out a mechanism for the reaction.
4.9* ALKYNE ANALOGS
Alkyne analogs based on Si, Ge, and Sn are yet another exotic class of group 14 compounds
with low coordination numbers. So far, they haven’t proved particularly useful, but they are
so “cool” in terms of their structures, bonding, and reactivity that they deserve at least a
brief mention. Once again, highly sterically hindered aryl groups such as i-Pr Ar proved
4
critical to their synthesis. The discussion here focuses on a digermyne (Stender, M., et al.
Angew. Chem. Int. Ed. 2002, 41, 1785–1787), which has been synthesized in two steps, as
shown below:
Cl Cl
− LiCl
Ge O O + i-Pr Ar Li i-Pr Ar Ge
4
4
Cl
(4.53)
Cl Ari-Pr 4
2 K
2 i-Pr Ar Ge Ge Ge
4
− 2 KCl
i-Pr Ar
4
The most distinctive structural feature of the heavier alkyne analogs is that, unlike typical
triple-bonded carbon atoms, the group 14 elements are not linearly coordinated. For the
digermyne, the bent geometry suggests that the Ge–Ge interaction is not a true triple bond,
but that the molecule might be better viewed as a bis(germylene):