Page 139 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 139
4.8 CARBENE AND ALKENE ANALOGS 119
The reactions can be broadly classified into two categories. The products shown in green
arise via a cycloaddition reaction; the others form by oxidative addition. Note that the steric
protection afforded by the trimethylsilyl group even allows the formation of a trisilaallene
(the product at the bottom).
REVIEW PROBLEM 4.15*
Assume that the oxidative additions (shown in black) and the cycloadditions (shown
in green) depicted above (reaction set 4.52) are concerted one-step processes. Use
arrow pushing to rationalize one reaction from each of the two categories.
REVIEW PROBLEM 4.16*
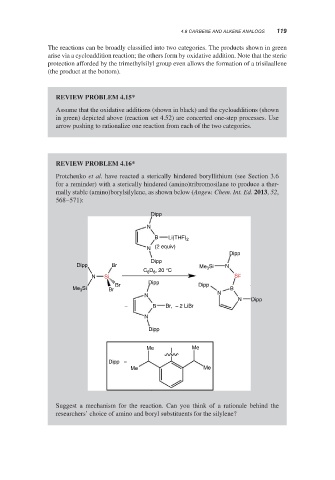
Protchenko et al. have reacted a sterically hindered boryllithium (see Section 3.6
for a reminder) with a sterically hindered (amino)tribromosilane to produce a ther-
mally stable (amino)borylsilylene, as shown below (Angew. Chem. Int. Ed. 2013, 52,
568–571):
Dipp
N
B Li(THF) 2
N (2 equiv)
Dipp
Dipp
Dipp Br Me Si N
C D , 20 °C 3
6 6
N Si Si
Dipp
Br Dipp
Me 3 Si Br B
N N
N Dipp
− B Br, − 2 LiBr
N
Dipp
Me Me
Dipp =
Me Me
Suggest a mechanism for the reaction. Can you think of a rationale behind the
researchers’ choice of amino and boryl substituents for the silylene?